Method for the in-situ recalibration of a comparison electrode incorporated into an electrochemical system
A technique for comparing electrodes and recalibrating, applied in the field of electrochemical systems, which can solve problems such as danger, voltage measurement drift, and component degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
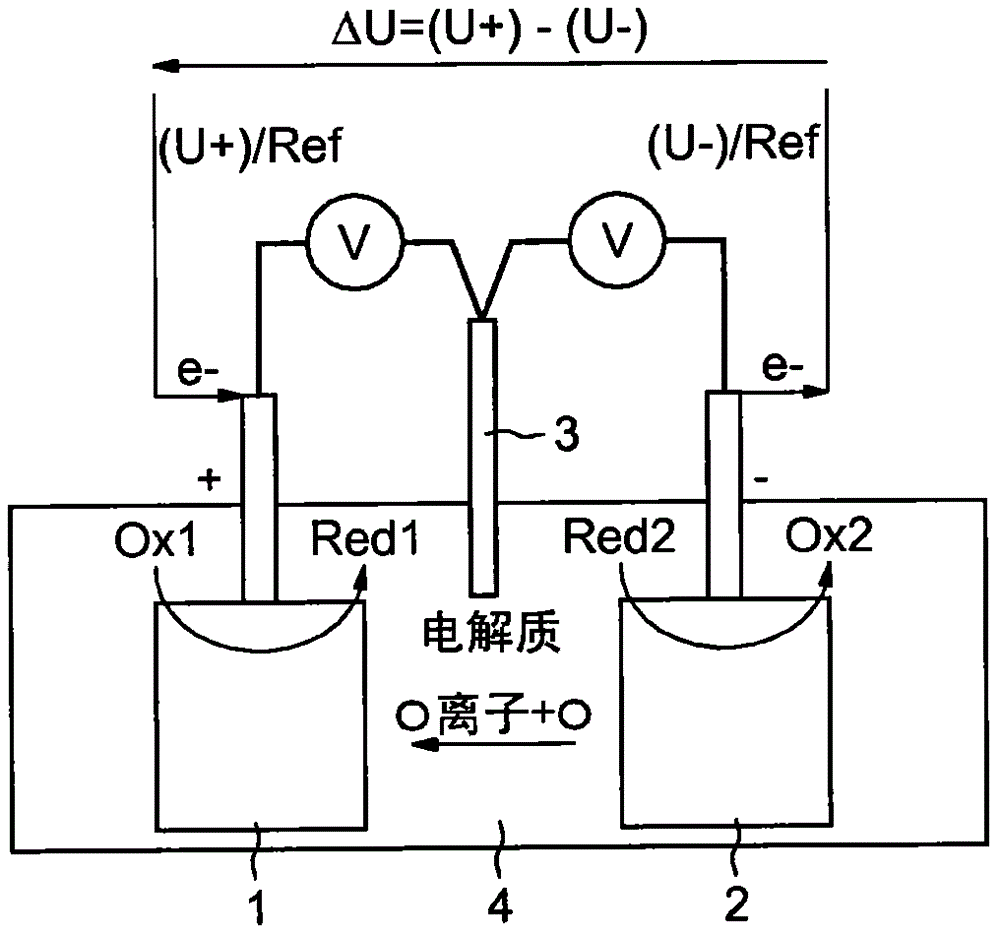
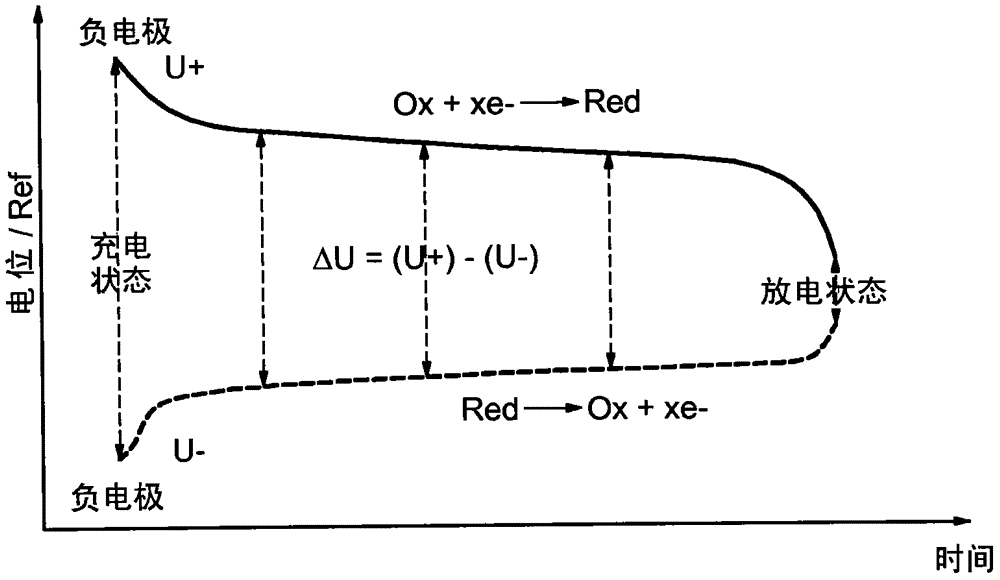
[0072] Figure 1a The electrochemical system shown comprises a working electrode 1 , a counter electrode 2 and a comparison electrode 3 all immersed in an electrolyte 4 . Electrolyte 4 may be liquid or solid. The voltage ΔU available during discharge depends on the difference between the potentials U+ and U- at the terminals of the working and counter electrodes. It can also be found that the potentials U+ and U− at the terminals of the working and counter electrodes are measured relative to the comparison electrode 3 . Figure 1b The evolution of the potential of the working electrode and of the counter electrode as a function of the state of charge and discharge of the accumulator, as well as the state of oxidation or reduction of these electrodes is shown in itself.
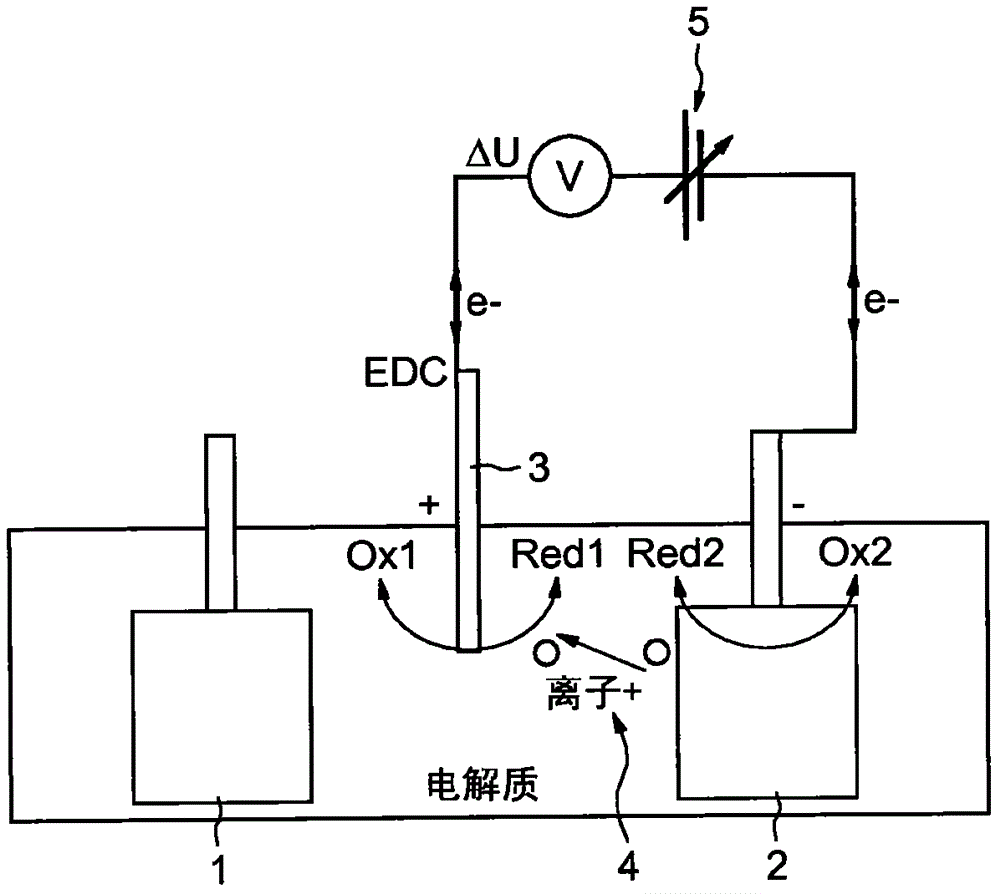
[0073] Embodiments of the invention will now be described that relate to an in situ recalibration method of a comparison electrode (EDC) integrated in an electrochemical system. The technology involved is mo...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap