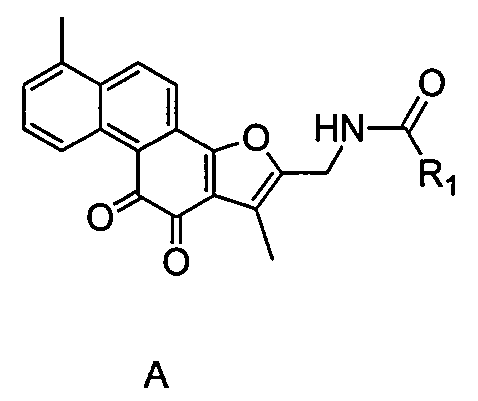
Tanshinone derivative, preparation method and application thereof
A technology of tanshinone and compound, applied in the field of novel tanshinone derivatives, can solve the problems of absorption, transport limitation, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
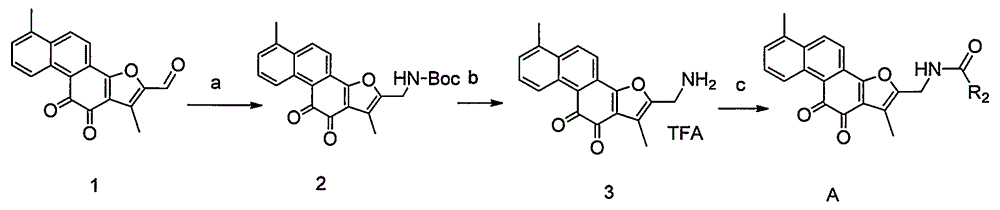
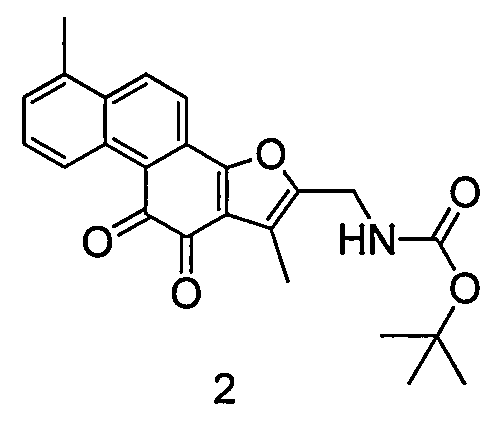
[0019] Example 1. Synthesis of compound 2
[0020]
[0021] Dissolve 5 g of raw material tanshinone I-2-formaldehyde and 2 g of Boc-amine in 50 ml of dry acetonitrile solution, add 4 g of triethylsilane at room temperature, add 2 ml of trifluoroacetic acid, and react under nitrogen protection, stirring at 25 degrees for 16 hours, The reaction solution was poured into 200ml saturated sodium bicarbonate solution, extracted twice with 100ml dichloromethane, the combined dichloromethane layers were washed twice with 200ml water, once washed with 200ml brine, dried, and concentrated under reduced pressure. The crude product was chromatographed on a silica gel column to obtain 5.3 g of Compound 2. LC-Ms: ESI: 406.2
[0022] 1 H NMR (300MHz, CDCl 3 )δ9.02(d, J=8.2Hz, 1H), 7.60(d, J=8.8Hz, 1H), 7.50-7.45(m, 1H), 7.30-7.24(m, 2H), 6.72(s, 1H ), 4.43(d, J=4.8Hz, 2H), 2.20(s, 3H), 2.18(s, 3H), 1.32(s, 9H)
example 2
[0023] Example 2. Synthesis of compound 3
[0024]
[0025] 5 g of compound 2 was dissolved in 50 ml of dichloromethane, 15 ml of trifluoroacetic acid was added dropwise in an ice bath, stirred at 20 degrees Celsius for 5 hours, and concentrated under reduced pressure to obtain 5.1 g of compound 3. The product was directly used in the next reaction without purification.
example 3
[0026] Example 3: Synthesis of Compound 4
[0027]
[0028] Add 100 mg of compound 3 and 100 mg of triethylamine to anhydrous dichloromethane, add 50 mg of acetic anhydride dropwise in an ice bath, and stir at room temperature for 0.5 hours. The reaction solution was washed once with saturated brine, dried, and concentrated under reduced pressure. The crude product was subjected to silica gel column chromatography to obtain 92 mg of product 4. LC-Ms: ESI: 348.2
[0029] 1 H NMR (300MHz, CDCl 3 )δ9.09(d, J=8.8Hz, 1H), 7.64(d, J=8.7Hz, 1H), 7.55-7.46(m, 1H), 7.32-7.24(m, 2H), 6.77(s, 1H ), 4.46(d, J=4.8Hz, 2H), 2.45(s, 3H), 2.23(s, 3H), 2.18(s, 3H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap