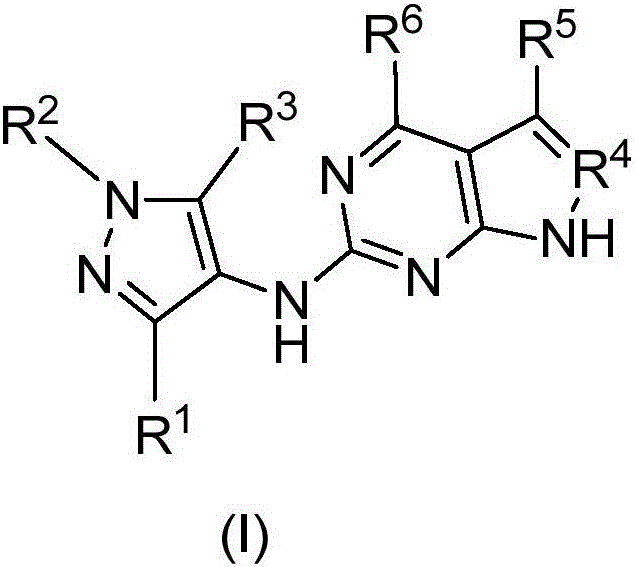
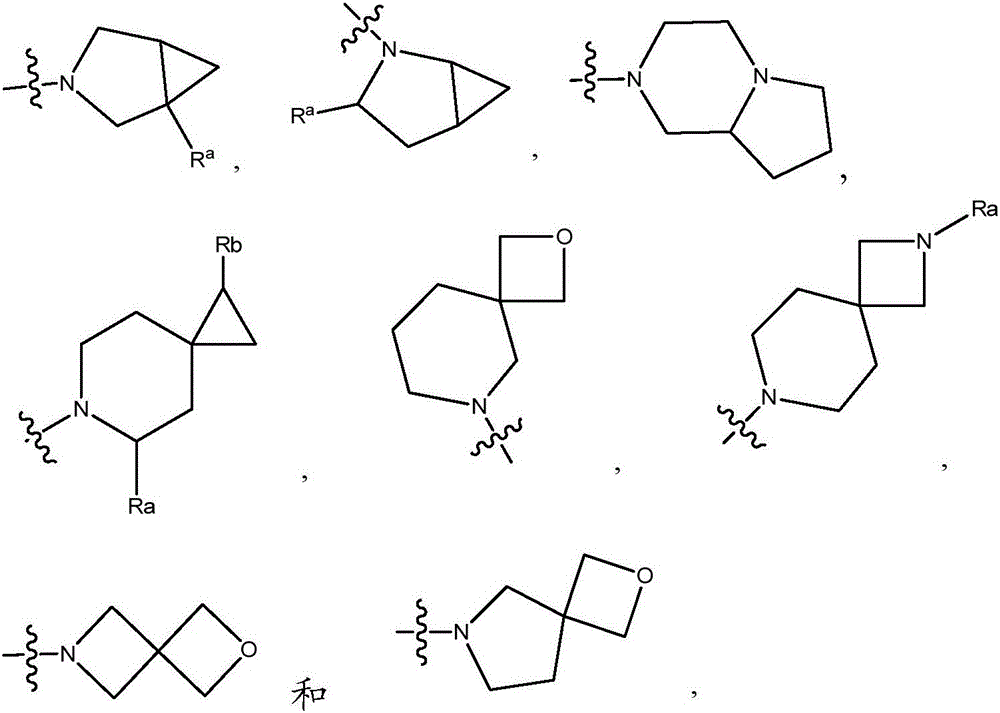
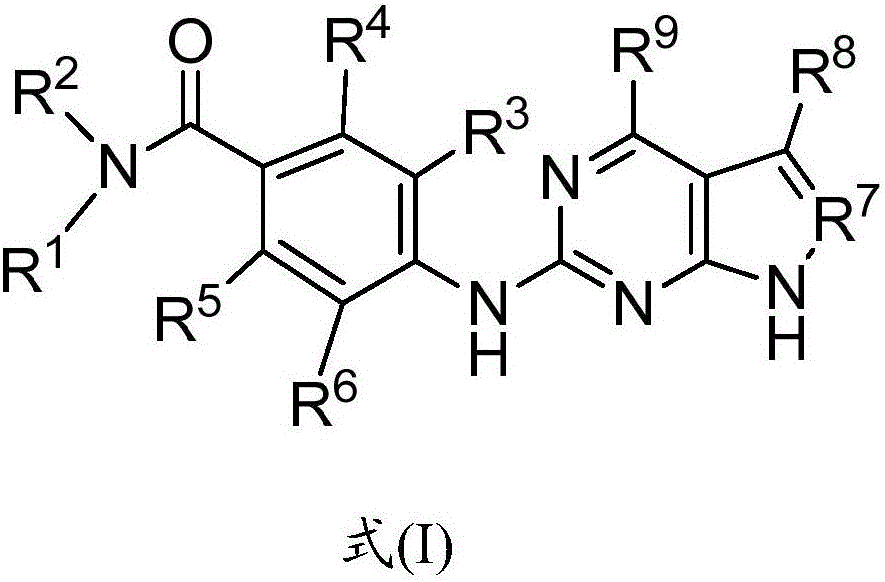
Compounds
A chemical compound and pharmaceutical technology, applied in the field of chemical compounds, can solve problems affecting cells to degrade α-synuclein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0560] (4-((4-ethoxy-7H-pyrrolo-[2,3-d]-pyrimidin-2-yl)amino)-3-methoxyphenyl)(morpholino)methanone (E1 )
[0561]
[0562] The title compound E1 was prepared according to general procedure 5 of the Buchwald reaction starting from 2-chloro-4-ethoxy-7H-pyrrolo-[2,3-d]pyrimidine (which can be prepared according to D1) and ( 4-Amino-3-methoxyphenyl)(morpholino)methanone (which can be prepared according to the procedure described in PCT International Application WO2012097682) as 810 mg of off-white solid. Yield: 35.4%.
[0563] LCMS:398[M+H] + . t R = 3.420 minutes. (LCMS condition 1)
[0564] 1 H NMR (400MHz, DMSO-d 6 ): δ8.62(d, J=8.0Hz, 1H), 7.63(s, 1H), 6.93-7.15(m, 3H), 6.34(d, J=3.5Hz, 1H), 4.52(q, J= 7.0Hz, 2H), 3.94(s, 3H), 3.45-3.70(m, 8H), 1.41(t, J=7.0Hz, 3H).
Embodiment 2
[0566](4-((4-ethoxy-7H-pyrrolo-[2,3-d]-pyrimidin-2-yl)amino)-2-fluoro-5-methoxyphenyl)(morpholino) Methyl ketone (E2)
[0567]
[0568] The title compound E2 was prepared according to general procedure 5 of the Buchwald reaction starting from 2-chloro-4-ethoxy-7H-pyrrolo-[2,3-d]pyrimidine (which can be prepared according to D1) and ( 4-amino-2-fluoro-5-methoxyphenyl)(morpholino)methanone (which can be prepared according to the procedure described in Journal of Medicinal Chemistry, 55(22), 9416-9433; 2012), It was 125 mg of an off-white solid. Yield: 47.1%.
[0569] LCMS:416[M+H] + . t R = 2.999 minutes. (LCMS condition 1)
[0570] 1 H NMR (400MHz, DMSO-d 6 ): δ11.50-11.83 (m, 1H), 8.59 (d, J=12.5Hz, 1H), 7.72 (s, 1H), 7.10 (broad singlet, 1H), 7.01 (d, J=6.1Hz, 1H), 6.36 (broad singlet, 1H), 4.52 (q, J=7.1Hz, 2H), 3.93 (s, 3H), 3.50-3.70 (m, 6H), 3.37-3.43 (m, 2H), 1.41 (t, J = 7.0 Hz, 3H).
Embodiment 3
[0572] (4-((4-ethoxy-7H-pyrrolo-[2,3-d]-pyrimidin-2-yl)amino)-3-methoxyphenyl)(4-methylpiperazine-1 -yl)-methanone (E3)
[0573]
[0574] (4-((4-ethoxy-7-tosyl-7H-pyrrolo-[2,3-d]-pyrimidin-2-yl)amino)-3-methoxyphenyl)(4 A solution of -methylpiperazin-1-yl)-methanone (which can be prepared according to D137) (100 mg, 0.177 mmol) and sodium hydroxide (2 mL, 4.00 mmol, 2M in water) in isopropanol (5 mL) Stir overnight at 60°C. The mixture was evaporated and 2N HCl was added until pH=7. The mixture was extracted with EA. The organic layer was washed with MgSO 4 Dry and evaporate. The residue was purified by prep-HPLC to afford the title compound E3 (25 mg, 0.061 mmol, 34.4% yield) as a white solid.
[0575] LCMS:411[M+H] + . t R = 1.259. (LCMS condition 2)
[0576] 1 H NMR (400MHz, DMSO-d 6 ): δ11.58 (broad singlet, 1H), 8.60 (d, J=8.3Hz, 1H), 7.63 (s, 1H), 6.93-7.13 (m, 3H), 6.33 (broad singlet, 1H), 4.51(d, J=7.0Hz, 2H), 3.93(s, 3H), 3.52(broad singlet, 4H), 2.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com