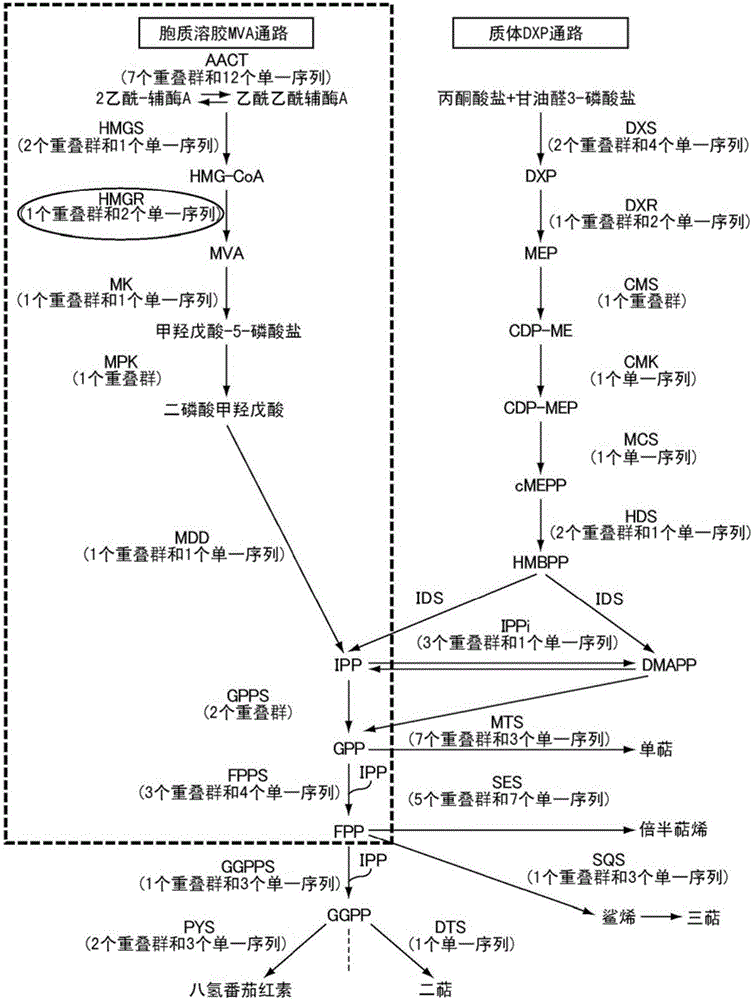
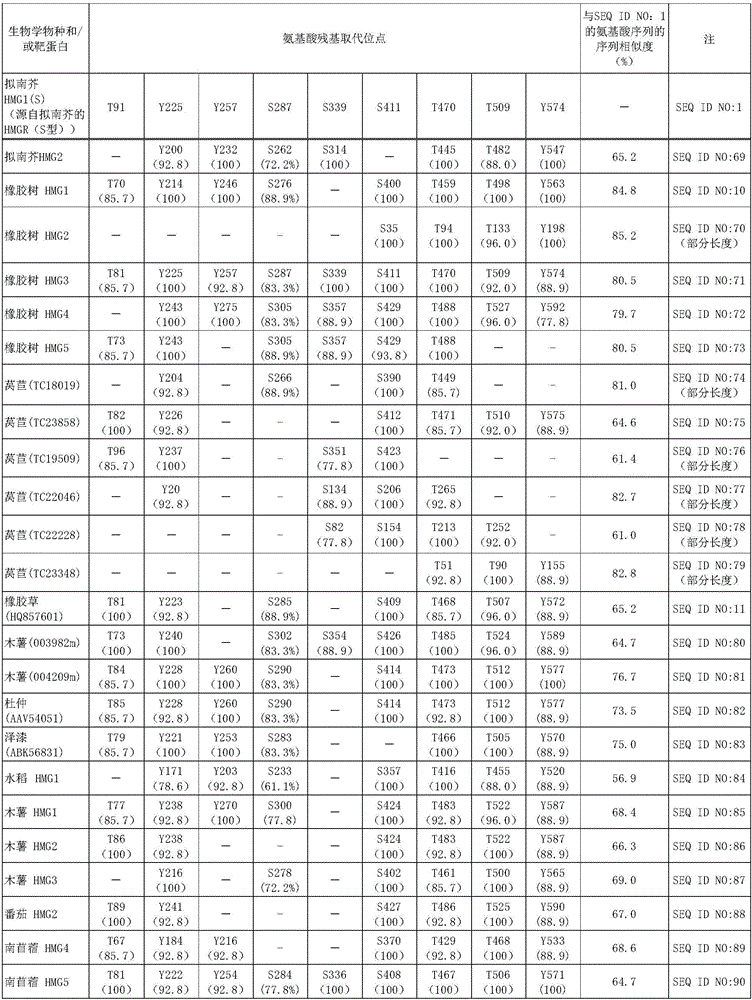
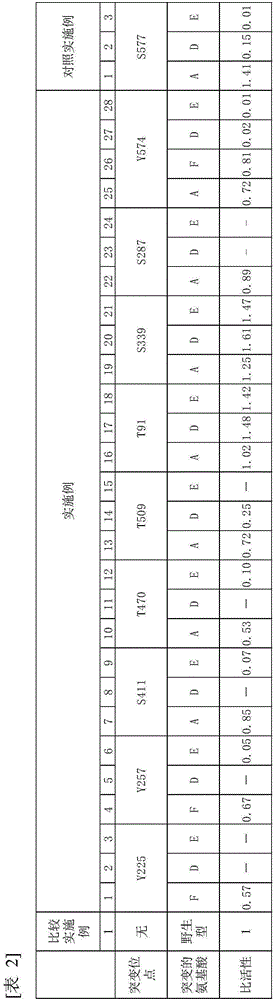
Mutein, method for producing said mutein, and gene encoding said mutein
A protein and gene technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0129] The present invention will be explained in detail with reference to examples, but the present invention is not limited to these examples.
[0130] (Suggestive evidence for the presence of new phosphorylation sites)
[0131] HMGR1 having a mutation in which serine 577 of Arabidopsis HMGR1 was replaced by alanine (hereinafter referred to as S577A-HMGR1) was transfected into an Arabidopsis mutant lacking endogenous HMGR1 to produce a mutant expressing S577A - Arabidopsis transformants of HMGR1 in which phosphorylation at serine 577 is known to reduce enzyme activity. ER fractions were collected from this transgenic plant expressing S577A-HMGR1 (HMGR is an ER-localized membrane protein). When the ER fraction containing S577A-HMGR1 was treated with phosphatase, its enzymatic activity was found to increase in the same manner as when the ER fraction containing wild-type HMGR from Arabidopsis expressing wild-type HMGR1 was treated with phosphorylated after enzyme treatment. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com