Selective inhibitors that interfere with the interaction of fibroblast growth factor receptor and Frs2 for the prevention and treatment of cancer and other diseases
A technology selected from, pharmaceutically acceptable salts, applied in the field of selective inhibitors that interfere with the interaction of fibroblast growth factor receptor and FRS2 for the prevention and treatment of cancer and other diseases, which can solve the weak effect of FRS2, reduced efficiency, problems such as increased toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
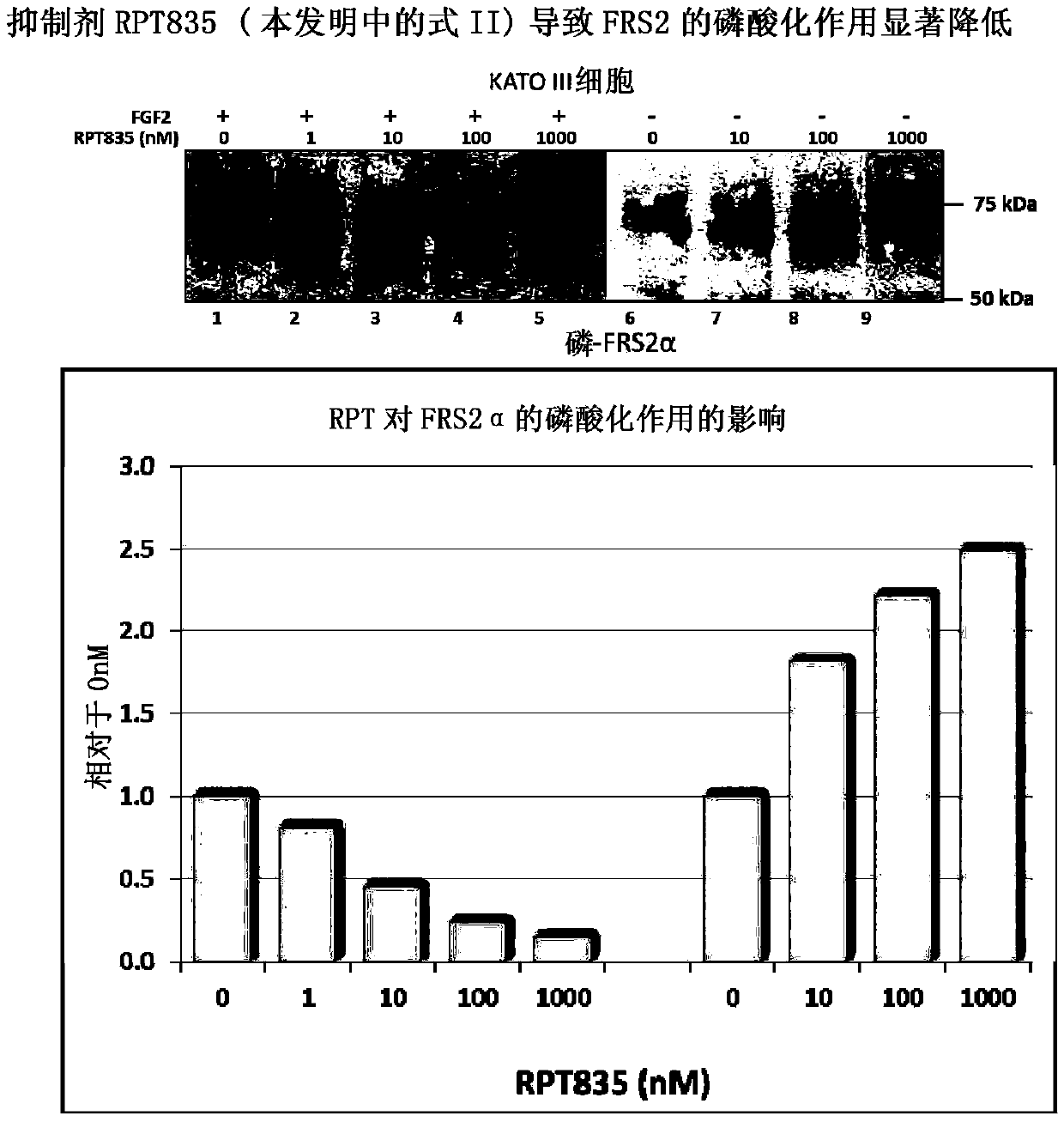
[0074] Example 1 (research results): Interference with FGFR2 and FRS2 interaction when inhibitor RPT835 is added
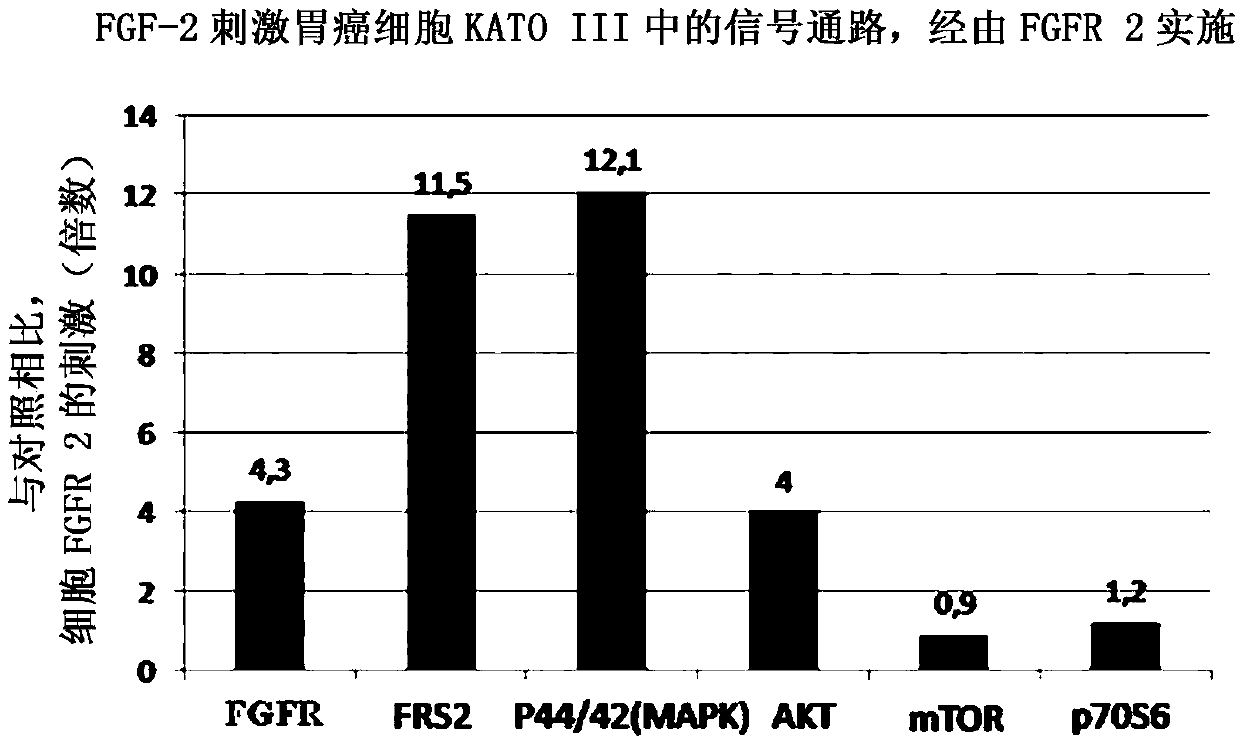
[0075] In order to evaluate the effect of the RPT835 inhibitor characterized by formula II of the present invention, gastric cancer cell KATO III expressing FGFR2 was used for FRS2 phosphorylation. FGFR was stimulated by adding FGF-2 at a concentration of 1 mg / mL and heparin at 10 mg / mL to the cells. RPT835 was added at various concentrations. A portion of the cells was left without the addition of the inhibitor, and a portion of the cells—without the addition of the inhibitor, or stimulator (control group).
[0076] Two standard methods for assessing the level of phosphorylation of FGFR and FRS2:
[0077] - Western blotting using manual work;
[0078] - Use automatic system Wes TM (ProteinSimple; Santa Clara, CA) automated capillary electrophoresis and immunodetection.
[0079] Stimulation of KATO III cells with fibroblast growth factor resulted in significa...
Embodiment 2
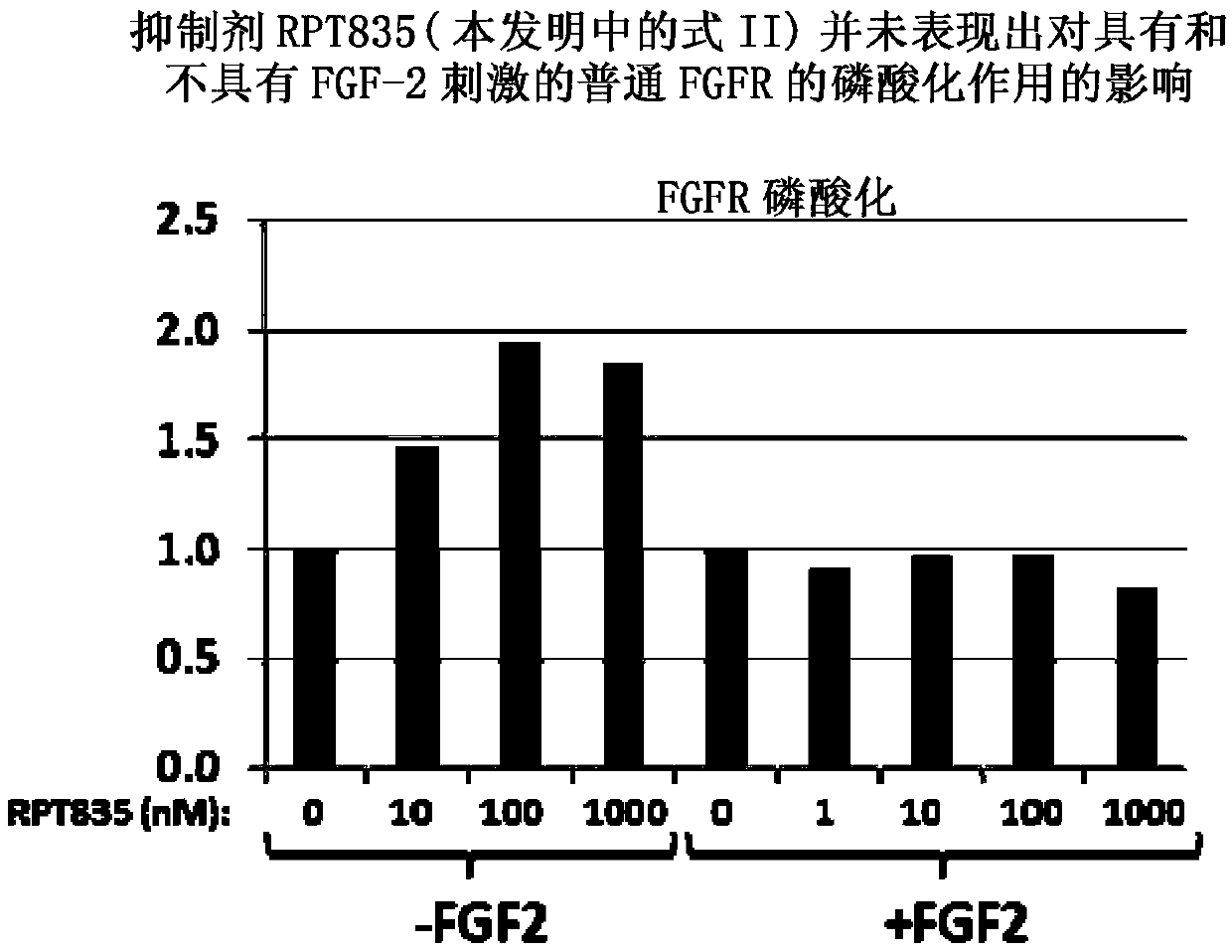
[0082] Example 2 (research results): RPT835 inhibitors do not affect the tyrosine kinase activity of FGFR.
[0083] The previous examples demonstrated the lack of effect of the inhibitor RPT835 on the phosphorylation of FGFR. In the present study, to disprove the mechanism of action of RPT835 as a tyrosine kinase inhibitor, the levels of common FGFR and phospho-FGF were evaluated before and after the addition of RPT835.
[0084] As in Example 1, gastric cancer cell KATO III, which strongly expresses FGFR2, was used. A portion of the cells was stimulated with FGF-2 at concentrations of 1 mg / mL and heparin at 10 mg / mL. Another portion of cells was left unstimulated. Inhibitor RPT835 was added to the cells at doses of 1, 10, 100, 1000 nM. A control group was kept without adding RPT835.
[0085] Using automated systems with immunoassays and using Wes TM (ProteinSimple; Santa Clara, CA) standard immunoblotting and automated capillary electrophoresis to assess the level of FGFR...
Embodiment 3
[0087] Example 3 (research results): Inhibitor RPT835 does not inhibit the binding of FGF-2 and FGFR2
[0088] A non-radioactive enzyme-linked FGF2 binding assay test was performed to evaluate whether RPT835 affects the binding of FGFR2 and ligand (FGF-2). In the binding assay of FGF-2, a recombinant chimeric protein FGFR2-Fc consisting of the human extracellular domain FGFR2α(IIIc) and the Fc fragment of human IgG1 was used. FGFR2α contains all 3 immunoglobulin-like domains and represents domain IIIс FGFR2 with high affinity for FGF-2. A 96-well plate was coated with FGFR2-Fc protein, and the following were added to the wells:
[0089] 1) FGF-2 only
[0090] 2) FGF-2+heparin
[0091] 3) Various concentrations of inhibitor RPT835+FGF-2+heparin
[0092] 4) A part of the wells retain no stimulator (FGF-2+heparin) and RPT835 (negative control).
[0093]After incubation, ELISA test using the corresponding antibody and subsequent determination of OD 650 (optical density at a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com