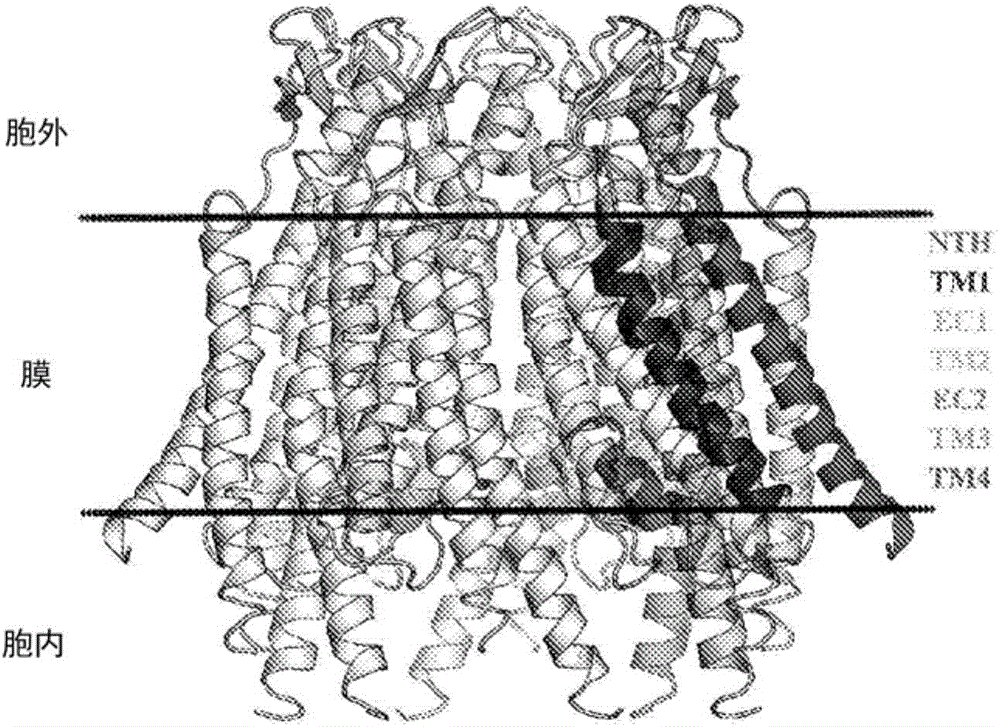
Specific modulators of connexin hemichannels
A connexin, specific technology, applied in the method, the field of compounds identified under this methodology, can solve the problem of no effective and specific identification and selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] In vitro test for blocking the opening / activation of hemichannels formed by connexins (26 or 43).
[0083] In vitro assays using HeLa cells transfected with connexin 26 or 42 tested the ability of different tracer molecules to assess the ability of compounds to reduce trafficking of these molecules.
[0084] Table 1: Inhibition rates against permeation tracers at a concentration of 5 μM
[0085]
[0086] The most potent inhibitor in vitro is Example 4, which has an IC of 20 nM 50 and 100% effectiveness, followed by Case 5, which has an IC of 50nM 50 and 100% effectiveness.
[0087] As a reference, ketone or glycyrrhetinic acid (CBX, ABG), which are widely used connexin blockers, showed an IC of 100 nM 50 and 100% effectiveness.
[0088] Accordingly, the inhibitors of the present invention exhibit appropriate opening / activation blocking activity of connexin 26 and 43 hemichannels in vitro.
Embodiment 2
[0089] Example 2: Use of compounds that block the activity of the hemichannel formed by connexin 26 or connexin 43.
[0090] The method comprises selectively or specifically contacting a hemichannel formed by connexin 26 or 43 with therapeutically effective amounts (in the nanomolar range) of at least two of the following compounds:
[0091] A: (R)-2-(4-chlorophenylfenyl)-2-oxo-1-furfurylethylquinoline-2-carboxylic acid ethyl ester,
[0092] B: (4-(4-chlorophenyl)piperazin-1-yl)propane,
[0093] C: acetic acid 2,2-bis([1,1'-biphenyl]-4-yl) acid,
[0094] D: (2R, 5S, 8R, 9S, 10S, 13S, 14S, 17S)-2-fluoro-10,13-dimethyl-3-oxohexadecahydro-1H-cyclopentadiene[a] Ethyl fluorene (fenantren)-17-benzoate,
[0095] E: (3S, 5S, 8R, 9S, 10S, 13S, 14S, 17S)-3-acetoxy-10,13-dimethylhexadecahydro-1H-cyclopentadien[a]fluorene (fenantren )-17-cyclohexanecarboxylate, F: (3S, 8R, 9S, 10R, 13S, 14S, 17R)-17-ethynyl-17-hydroxy-10,13-dimethyl-2,3, 4,7,8,9,10,11,12,13,14,15,16,17-Tetrahydro-1H-...
Embodiment 3
[0099] Example 3: Biological Evaluation
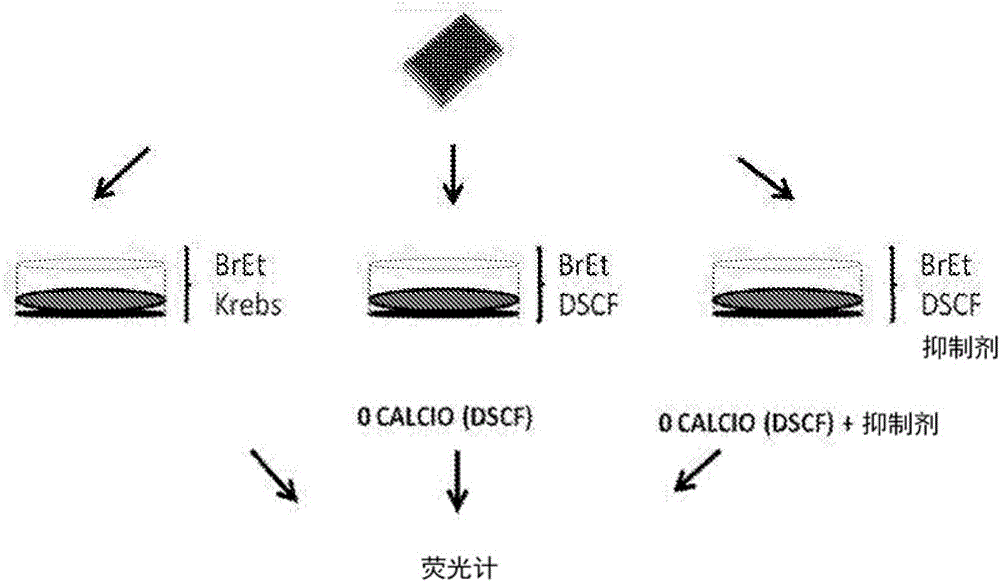
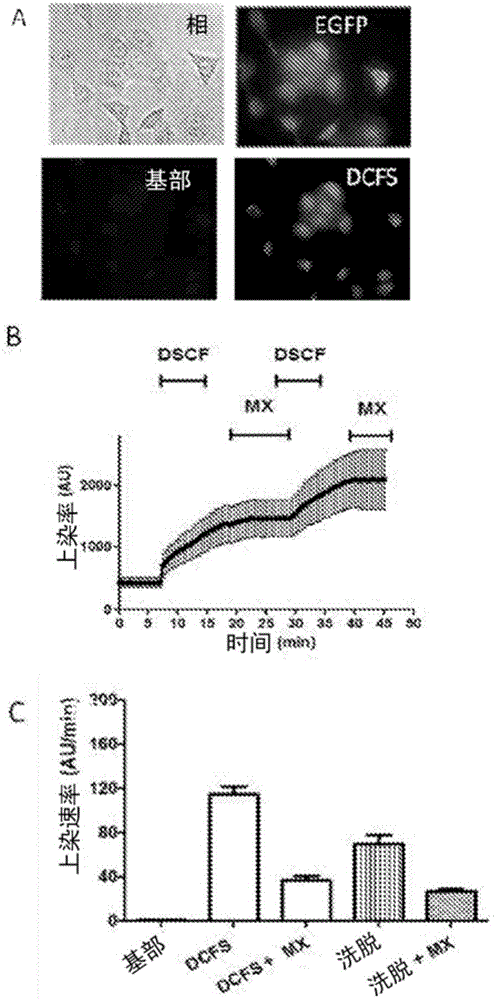
[0100] Mouse HeLa cells transfected with connexin 26 or 43 were used. To evaluate the activity of each compound against connexin hemichannels, 24 h before each experiment, 10 3 HeLa cells transfected with connexin were seeded in wells of a multi-well (90) plate. Then, with normal levels of divalent cations (Ca 2+ and Mg 2+ ) in a locke solution or a locke solution free of divalent cations, the locke solution also containing 5 μM ethidium bromide. Simultaneously, cells were treated with various concentrations of each candidate compound and incubated for 5 minutes.
[0101] As shown in Table 2, HeLa cells transfected with connexin 43 (Cx43) were seeded in multi-well (96) plates, and after 24 h, the cation-free solution was evaluated in the presence or absence of the compound in question ( Ethidine uptake in DCFS). Ethidine uptake was assessed by measuring excited fluorescence with a fluorometer. A compound corresponds to an inhibi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com