Bisphosphonate-containing pharmaceutical vaccine composition for humoral immunity
一种双膦酸盐剂、体液免疫的技术,应用在药物组合、含有效成分的医用配制品、非有效成分的医用配制品等方向,能够解决不能期待治疗效果等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
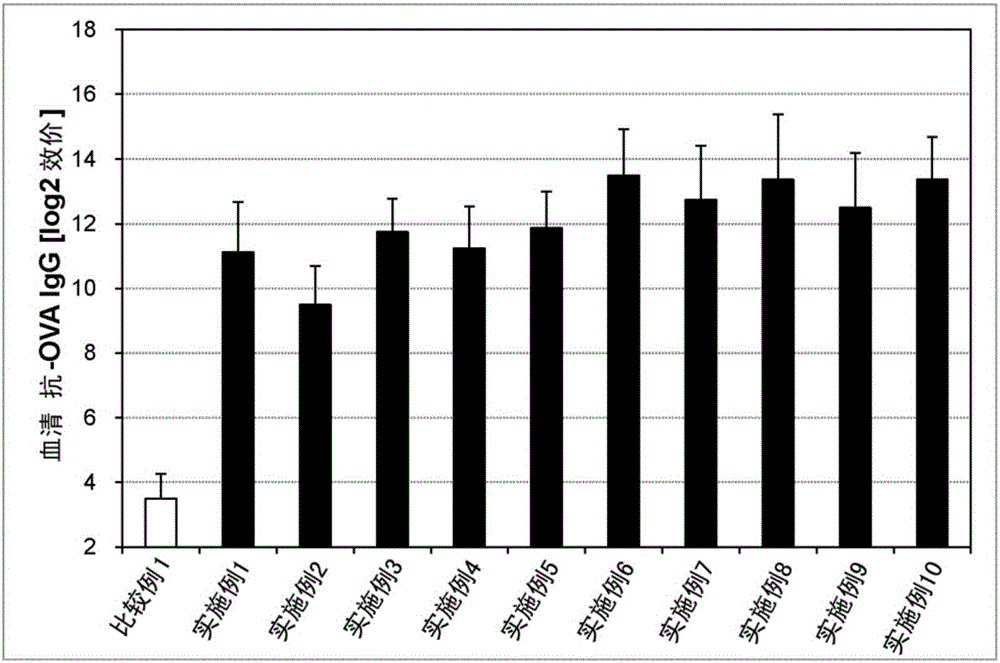
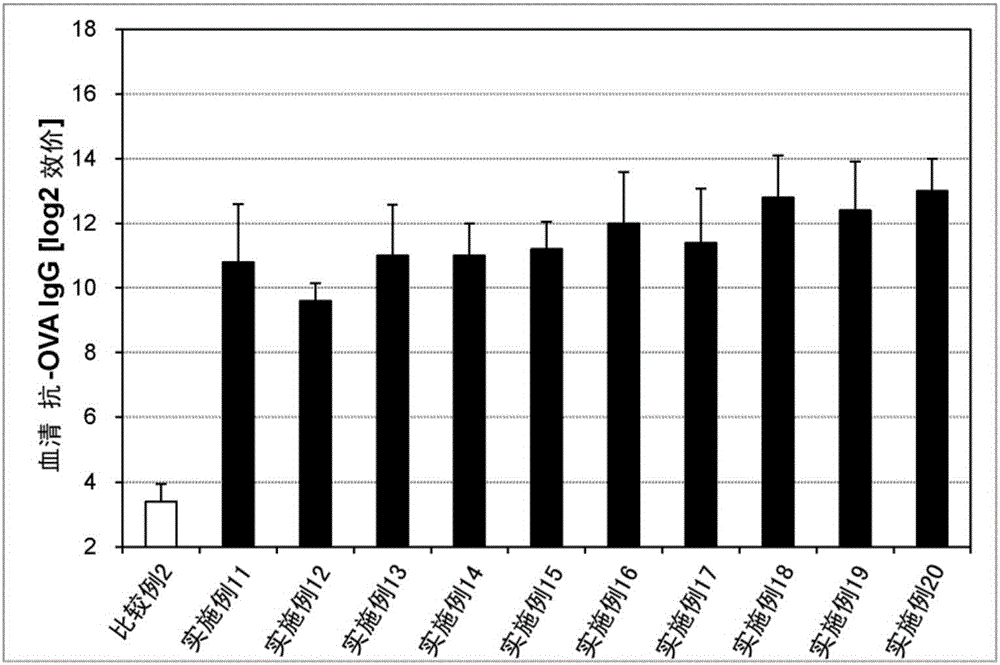
Embodiment 1~20、 comparative example 1、2
[0141] (Preparation of Liquid Preparations for Transmucosal Administration)
[0142] Liquid preparations for transmucosal administration (nasal administration or sublingual administration) having the compositions in Tables 1 and 2 below were prepared. Specifically, an antigen (ovalbumin (OVA), manufactured by Sigma-Aldrich Co., Ltd.) and an immunity-inducing promoter as a bisphosphonate were compounded in the compounding amounts shown in the following Tables 1 and 2, and Physiological saline and mixed to obtain a liquid preparation for transmucosal administration (nasal administration or sublingual administration).
[0143] As the immune induction enhancer of the bisphosphonate, etidronate (manufactured by LKT Laboratories), clodronate (manufactured by LKT Laboratories), tiludronate (manufactured by Sigma-Aldrich), Pamidronate (manufactured by Sigma-Aldrich), neridronate (manufactured by Sigma-Aldrich), alendronate (manufactured by Medichem), ibandronate (manufactured by URQU...
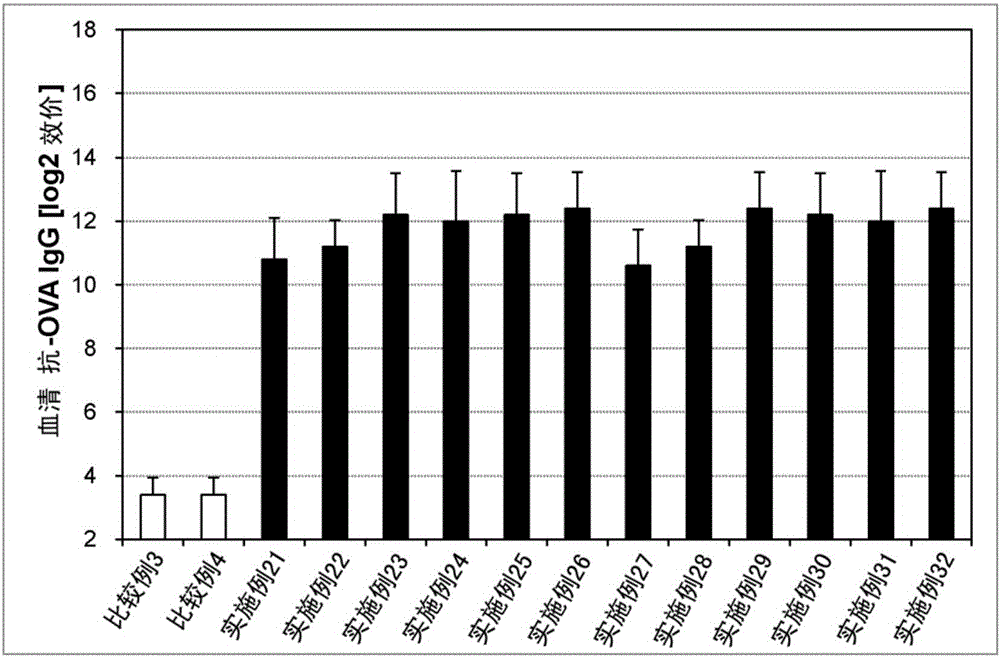
Embodiment 21~32、 comparative example 3、4
[0149] (Preparation of solid preparation for sublingual administration)
[0150] Solid preparations for sublingual administration (lyophilized preparations or film preparations) having the compositions in Table 3 below were prepared. Specifically, an antigen (ovalbumin (OVA), manufactured by Sigma-Aldrich), an immunity-inducing enhancer as a bisphosphonate agent, a hydroxyl Physiological saline was added to propylcellulose (HPC-SSL, manufactured by Nippon Soda Co., Ltd.) and mixed to obtain a preparation solution. Then, 25 mg of the preparation solution was dispensed and freeze-dried to obtain a freeze-dried preparation or dried under reduced pressure to obtain a film preparation. As the immunity induction enhancer of the bisphosphonate preparation, the same substance as that used for the preparation of the liquid preparation for transmucosal administration was used.
[0151] [table 3]
[0152]
[0153]
[0154] The following evaluations were performed on the liquid pr...
Embodiment 33~42、 comparative example 5
[0168] (Preparation of Cream for Transdermal Administration)
[0169] A cream for transdermal administration having the composition of Table 4 below was prepared. Specifically, an antigen (ovalbumin (OVA), manufactured by Sigma-Aldrich) and an immunity-inducing enhancer as a bisphosphonate agent were compounded in the compounding amounts shown in the following Table 4, and the base material was added thereto. (Base cream) The total amount was adjusted to 100 parts by weight, and mixed to obtain a cream for transdermal administration. The base cream used was prepared by compounding and kneading materials according to the composition described in Table 5.
[0170] As the immune induction promoter of the bisphosphonate preparation, the same substance as that used for the preparation of the liquid preparation for nasal cavity or sublingual administration was used. White petrolatum, sorbitan monostearate, isostearic acid, benzyl alcohol, stearyl alcohol, Tween 60, concentrated gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com