Vaccine pharmaceutical composition for suppressing apoptosis of ctl or inhibiting suppression of induction of ctl
A composition and drug technology, applied in the field of vaccine pharmaceutical compositions for inhibiting CTL apoptosis or blocking CTL induction inhibition, can solve problems such as inability to effectively induce antigen-specific CD8-positive T cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2
[0460] (Preparation of cream for transdermal administration)
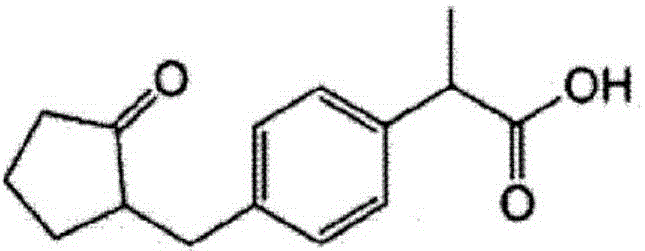
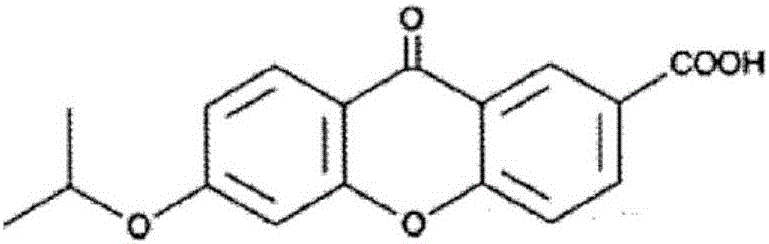
[0461] A cream for transdermal administration having the composition of Table 2 below was prepared. Specifically, the following antigen peptides, the first cellular immunity induction promoter, the second cellular immunity induction promoter as a helper peptide, and dimethylmethylene were mixed with the compounding amounts shown in Table 2. 15% by weight of sulfone (DMSO) was added to the base material (primer) to make the total 100% by weight and mixed to obtain a cream for transdermal administration. The primer used was prepared by mixing according to the composition compounding materials described in Table 1.
[0462] Prepare to laminate PET film / PET non-woven fabric (with area 0.7cm 2 ) A composite substrate obtained by bonding the PET film side as the tape side to the center of the fixing adhesive tape. 4 mg of a cream for transdermal administration was applied to the non-woven fabric portion of the composite sub...
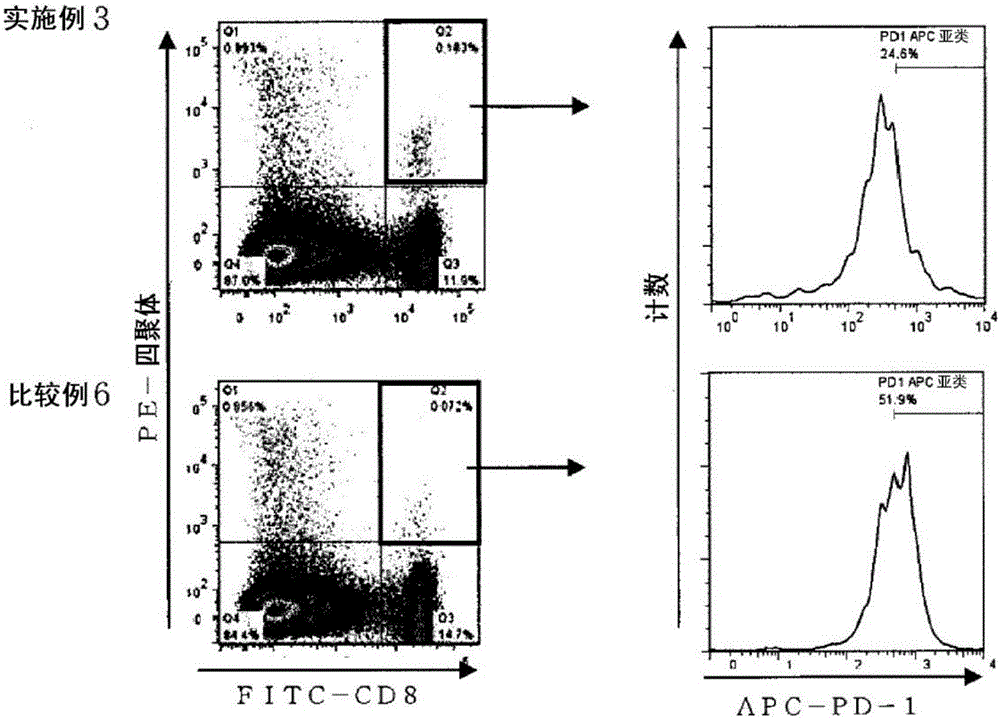
Embodiment 3~5、 comparative example 1~6
[0474] (Preparation of injection liquid preparation for subcutaneous administration)
[0475] A liquid injection preparation for subcutaneous administration having the composition shown in Table 2 below was prepared as a sample for immunization experiment. Specifically, the antigens shown above, the first cellular immunity induction promoter, and the second cellular immunity induction promoter as a helper peptide were weighed and mixed in the compounding amounts shown in Table 2, and physiological saline was added. Make 1000 μL and mix with a homogenizer to prepare an injection liquid preparation for subcutaneous administration.
[0476]
[0477] For the injection liquid preparations for subcutaneous administration obtained in the examples, the following evaluations were performed.
[0478] Mouse immunity test 2 (injection)
[0479] In accordance with the following procedure, a mouse immunization test was performed using the above-mentioned injection liquid preparation for subcutaneo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com