Composition for immunity induction promotion and vaccine pharmaceutical composition
A technology of immune induction and composition, which is applied in the field of immune induction and promotion composition and vaccine pharmaceutical composition, and can solve problems such as difficulty in administration technology, burden on patients, and unclear effective promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
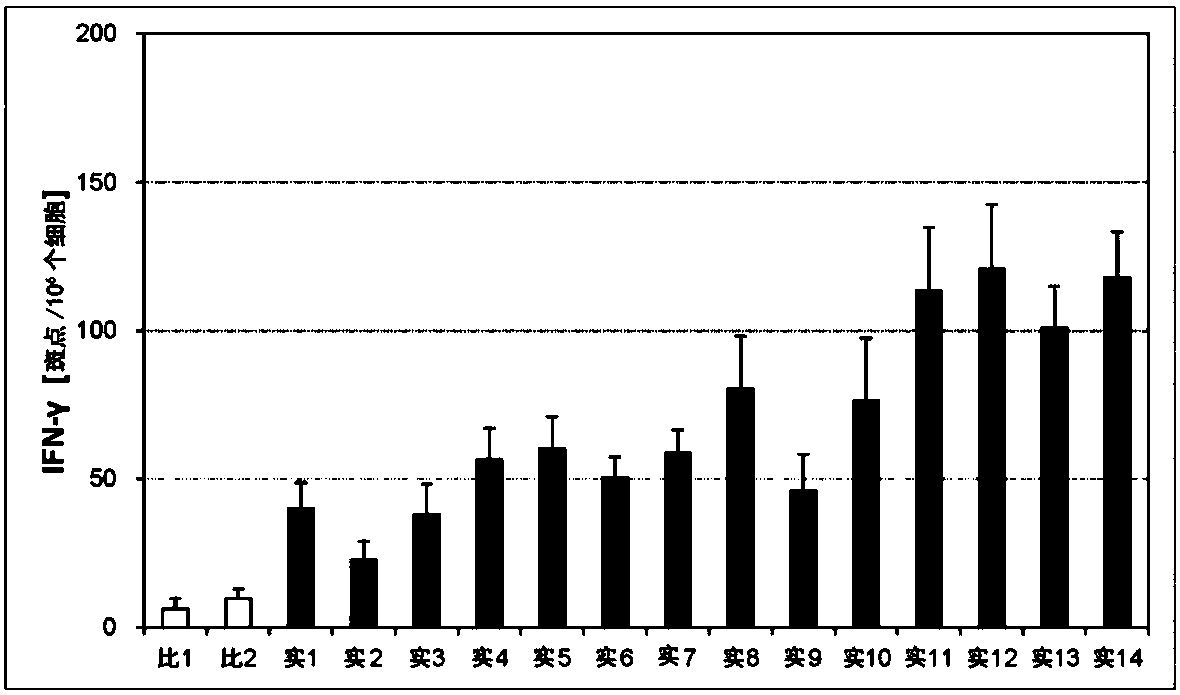
Embodiment 1~14、 comparative example 1~2
[0203] (Preparation of Cream for Transdermal Administration)
[0204] A cream for transdermal administration having the composition of Table 1 below was prepared.
[0205] Specifically, 5% by mass of the antigen shown below, 3% by mass of the first immunity-inducing promoter, 1% by mass of the second immune-inducing promoter, and two 15% by mass of methyl sulfoxide (DMSO), and a base (base cream) was added thereto to make the total amount 100% by mass, and mixed to obtain a cream for transdermal administration. The base cream used was prepared by compounding and kneading materials according to the compositions shown in Table 8. White petrolatum, sorbitan monostearate, isostearic acid, benzyl alcohol, stearyl alcohol, Tween 60, concentrated glycerin, and dimethyl sulfoxide (DMSO) were purchased from Wako Pure Chemical Industries, Ltd. Cetyl alcohol was purchased from Tokyo Chemical Industry Co., Ltd.
[0206] Prepare PET film / PET non-woven fabric laminate (area 0.7cm 2 ) A ...
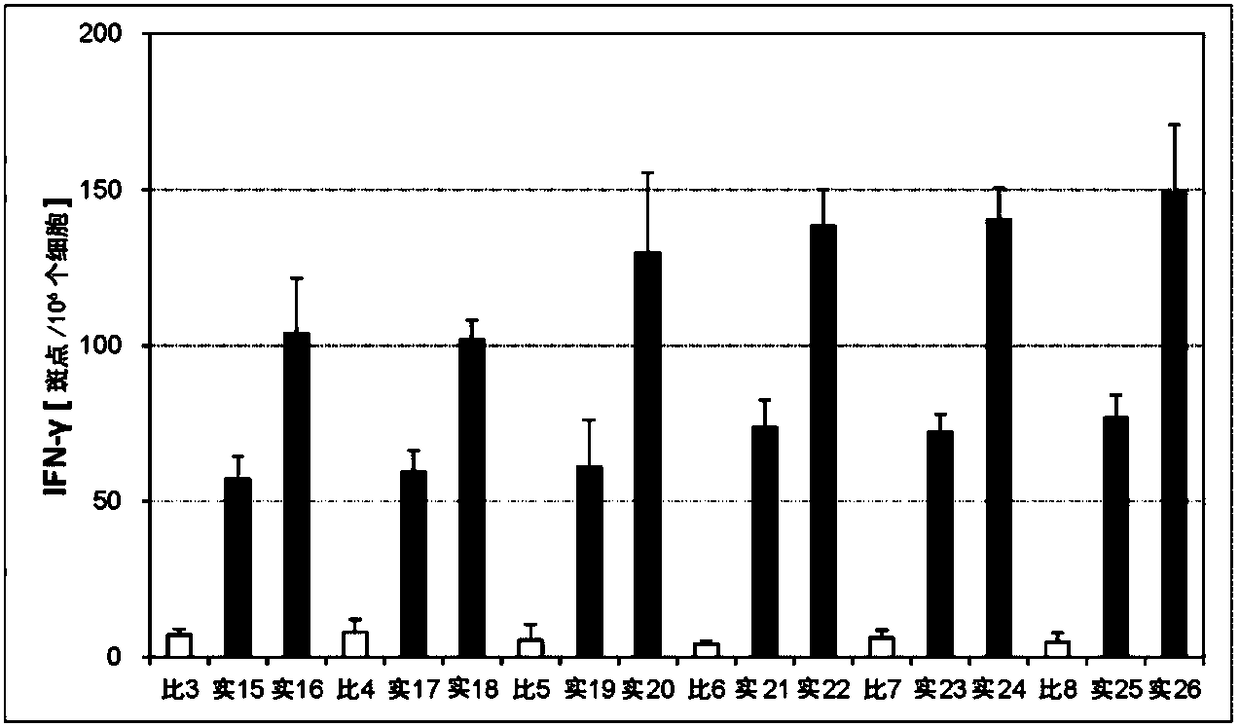
Embodiment 15~26、 comparative example 3~8
[0235] (Preparation of adhesive tape for transdermal administration)
[0236] Tape preparations for transdermal administration having the compositions in Table 2 below were prepared. Specifically, the antigen, the first immunity induction promoter, and the second immunity induction promoter were compounded in the compounding amounts shown in the following Table 2, and the components after drying with an organic solvent and the adhesive base The adhesive base shown in the following Table 2 and the organic solvent (ethyl acetate) were mix|blended and mixed so that the total may be 100 mass %, and the adhesive solution was prepared. The obtained pressure-sensitive adhesive solution was spread on a release liner so that the thickness after drying was about 80 μm, and the organic solvent was removed by drying to form a pressure-sensitive adhesive layer. As the release liner, a liner made of polyethylene terephthalate (PET) (thickness: 75 μm) subjected to a silicone release treatme...
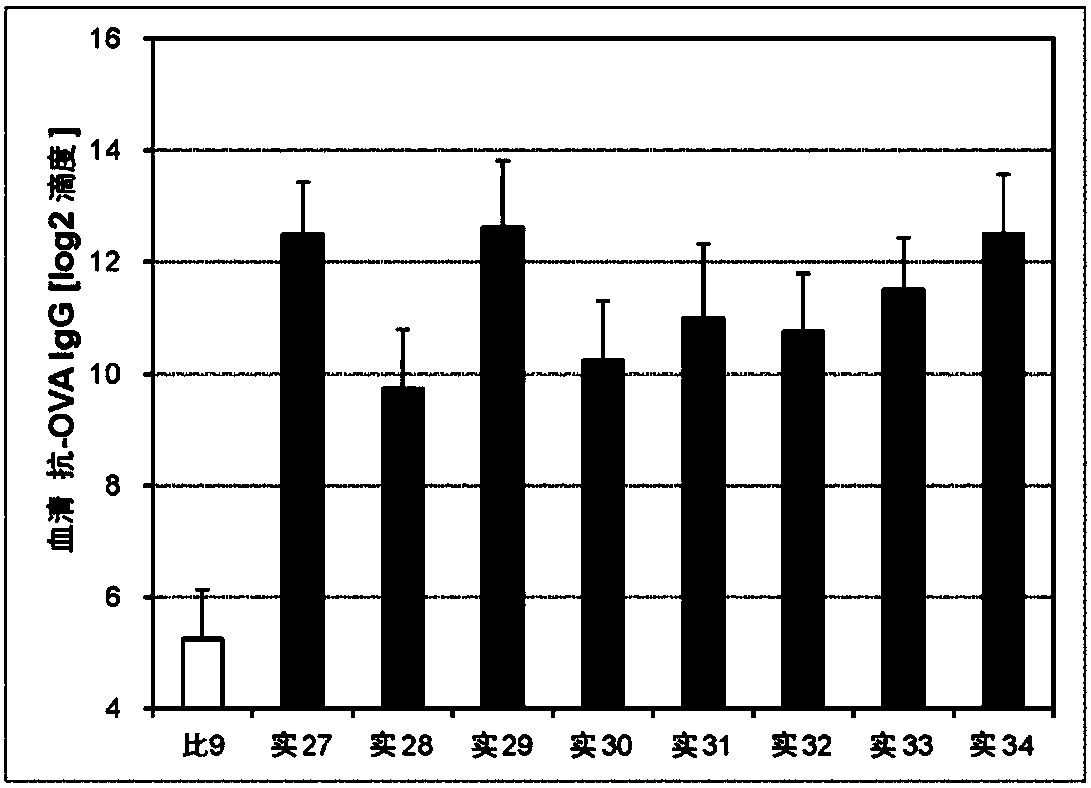
Embodiment 27~42、 comparative example 9~10
[0245] (Preparation of Liquid Preparations for Transmucosal Administration)
[0246] Liquid preparations for transmucosal administration (nasal administration or sublingual administration) having the compositions in Tables 3 and 4 below were prepared. Specifically, the antigen (ovalbumin (OVA)) and the first immunity induction promoter were compounded in the compounding amounts shown in the following Tables 3 and 4, physiological saline was added thereto, and 10 μL was prepared for nasal administration. Or in the case of sublingual administration, prepare 30 μL and mix to obtain a liquid preparation for transmucosal administration (nasal administration or sublingual administration).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com