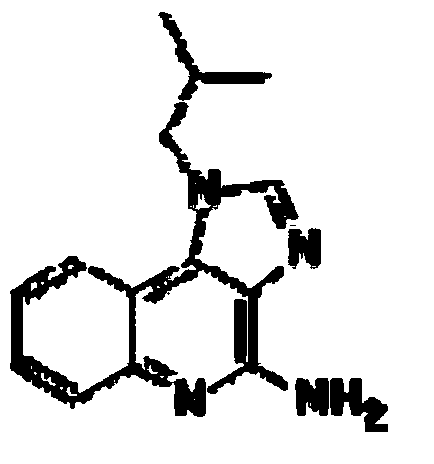
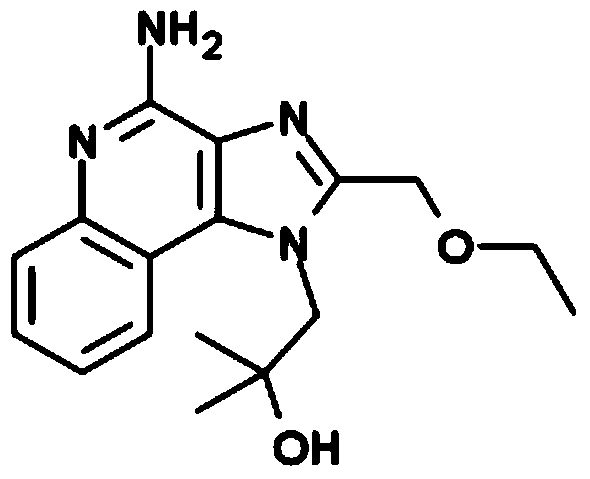
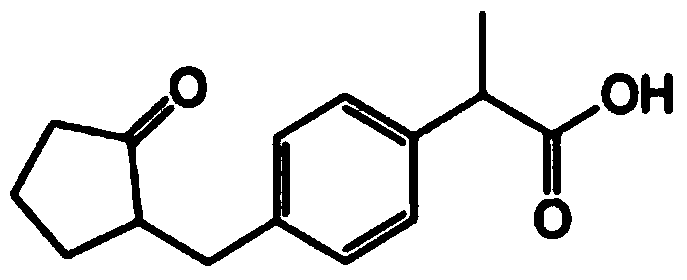
Vaccine composition for transdermal or mucosal administration
A technology of vaccine composition and mucosal administration, applied in drug combination, microorganism, drug delivery, etc., can solve the problems of heat-labile enterotoxin, administration to difficult-to-skilled medical workers, needle stick infection accident patients, etc., and achieve high cellular immunity induction effect, the effect of enhancing the effect of inducing cellular immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0406] Strip preparation for transdermal administration
[0407] The adhesive used in the tape preparation was prepared as shown below.
[0408] (Acrylic Adhesive A)
[0409] In an inert gas atmosphere, 75 parts of 2-ethylhexyl acrylate, 22 parts of N-vinyl-2-pyrrolidone, 3 parts of acrylic acid and 0.2 parts of azobisisobutyronitrile were carried out in ethyl acetate at 60 ° C. Solution polymerization was carried out to obtain an acrylic adhesive A solution.
[0410] (PIB rubber-based adhesive)
[0411] 24 parts of polyisobutylene (Opanol B200, manufactured by BASF Corporation), 36 parts of polyisobutylene (Opanol B12, manufactured by BASF Corporation), and 40 parts of alicyclic petroleum resin (ALCON P-100, manufactured by Arakawa Chemical Co., Ltd.) were dissolved in toluene , to obtain a PIB rubber-based adhesive solution.
[0412] Strip preparations having the compositions of the following Tables 1 to 3 were produced. Specifically, an adhesive solution, an antigeni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com