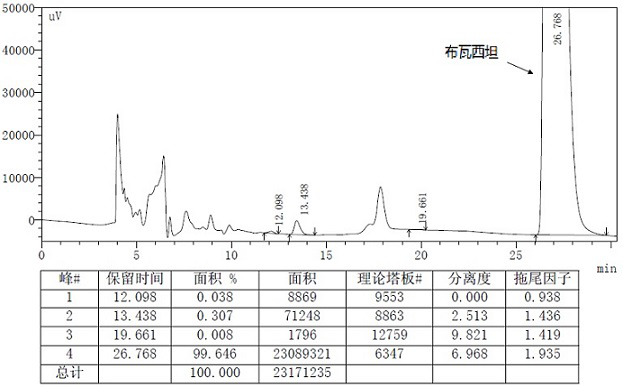
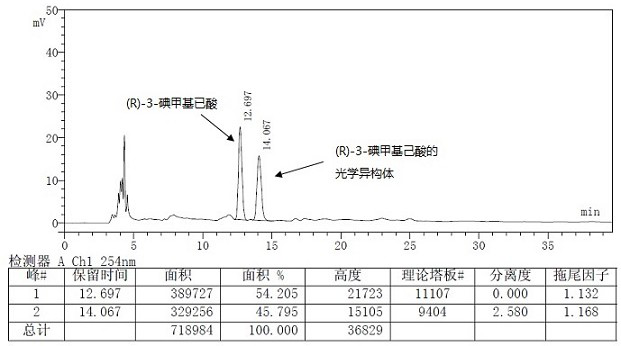
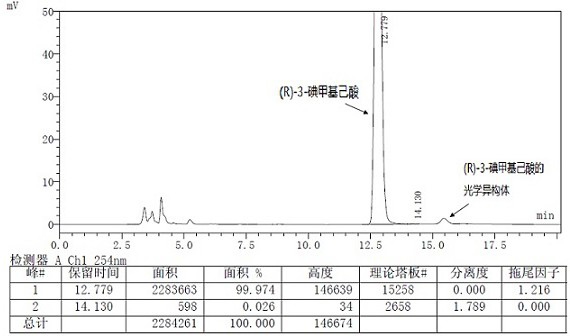
A kind of preparation method of buvaracetam and its intermediate
A compound and selected technology, applied in the direction of organic chemistry, can solve the problems of difficult operation, high substance content, difficult crystallization, etc., and achieve the effect of improving optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Dissolve 10 g of (R)-4-n-propyl-dihydrofuran-2-one (gas chromatography purity 85%, optical purity 95%) in acetonitrile, cool down to about -10°C, and dropwise add 8.4 g trimethyl Chlorosilane was added with 11.6g of sodium iodide, after the addition was complete, the reaction was monitored by TLC. After the reaction was complete, the reaction was quenched with dilute hydrochloric acid, extracted with DCM / water, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain (R)-3-iodomethyl n-hexanoic acid (optical purity 94.6%), directly used in the next step reaction. δ H (300 MHz, CDCl 3 ): 0.9-0.95(3H, m), 1.29-1.36 (4H, m), 1.71-1.74 (1H, m), 2.37-2.49 (2H,m),3.27-3.41 (2H, m), 11.07(1H ,s).
[0113] (R)-3-Iodomethyl n-hexanoic acid was dissolved in 20 ml of dichloromethane, and 36.8 g of phosphorus pentachloride was added at 10°C. The reaction was carried out at 10°C, and the rea...
Embodiment 2
[0116] Dissolve 10g of (R)-4-n-propyl-dihydrofuran-2-one (gas chromatography purity 90%, optical purity 98%) in cyclohexane, heat up to 70°C, add 7g hydrobromic acid dropwise , the addition was completed, and the reaction was monitored by TLC. After the reaction was complete, the reaction was quenched with dilute hydrochloric acid, extracted with DCM / water, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain (R)-3-bromomethyl-n-hexanoic acid (optical purity 97.3%), directly used in the next step reaction.
[0117] Dissolve (R)-3-bromomethyl n-hexanoic acid in 20ml of cyclohexane, and add 19.4g of oxalyl chloride at the boiling point of the solvent. The reaction was carried out at the boiling point of the solvent, and monitored by TLC. After the reaction was complete, it was concentrated to dryness under reduced pressure to obtain (R)-3-bromomethyl-n-hexanoyl chloride, which was dir...
Embodiment 3
[0120] Dissolve 10 g of (R)-4-n-propyl-dihydrofuran-2-one (gas chromatography purity 80%, optical purity 94%) in acetone, adjust the temperature to the boiling point of the solvent, and add 8.4 g of trimethyl Chlorosilane, add 23.2g of sodium iodide, after the addition is complete, TLC monitors the reaction. After the reaction was complete, the reaction was quenched with dilute hydrochloric acid, extracted with DCM / water, the organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain (R)-3-iodomethyl n-hexanoic acid (optical purity 93.41%), directly used in the next step reaction.
[0121] (R)-3-Iodomethyl n-hexanoic acid was dissolved in 20 ml of methylene chloride, and 62.4 g of thionyl chloride was added. The reaction was carried out at 80°C, and the reaction was monitored by TLC. After the reaction was complete, it was concentrated under reduced pressure to dryness to obtain (R)-3-iodomet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com