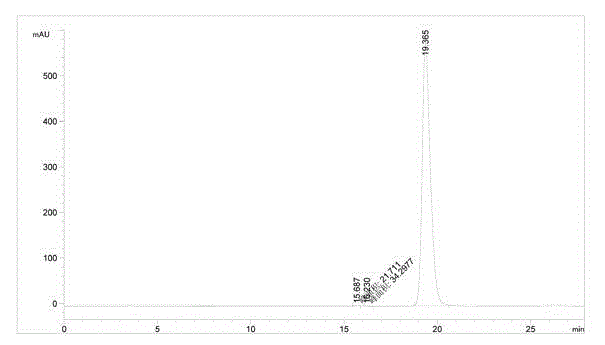
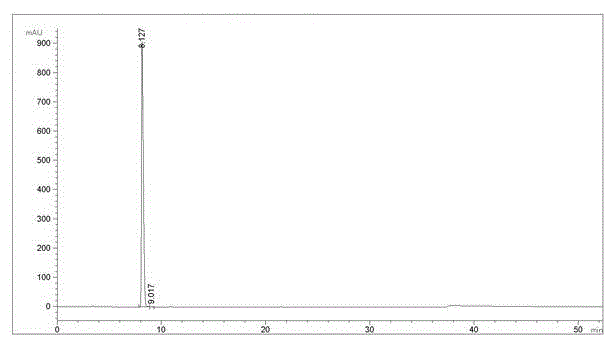
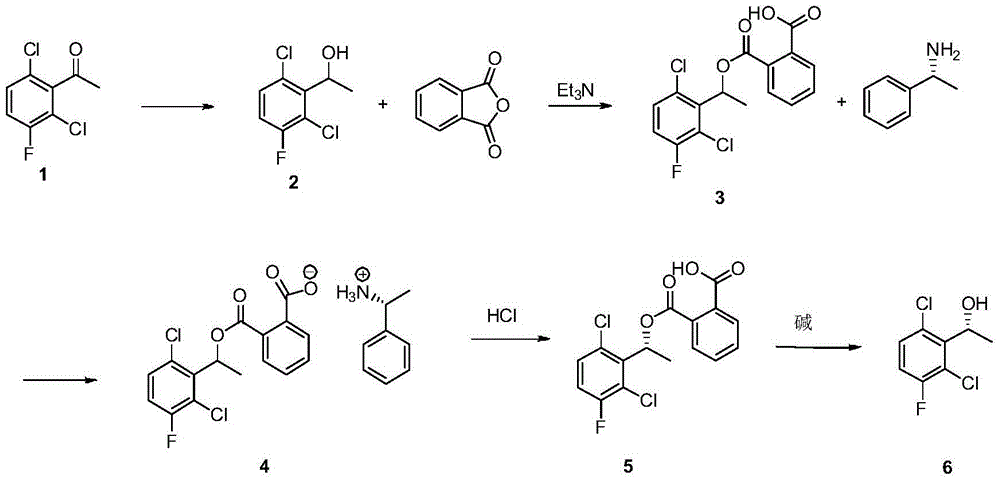
Preparation method of (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol
A technology of fluorophenylethanol and fluorophenyl, which is applied in the field of drug synthesis, can solve the problems of long steps, high ee% value, high yield, and low yield, and achieve the effects of improved purity, safe use, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Dissolve 174g of compound 1 in 500mL of ethanol, add 45g of sodium borohydride in batches at room temperature, stir at room temperature for about 2 hours after the addition is complete, spot the sample to determine the end point of the reaction, after the completion of the reaction, quench the reaction with 2N hydrochloric acid, and Adjust the pH to about 2, then extract four times with 2L ethyl acetate, wash the organic phase with saturated sodium carbonate solution and saturated sodium chloride solution respectively, dry, and remove the solvent to obtain a light yellow viscous liquid (compound 2). The rate is 96%, and the content is 98%.
Embodiment 2
[0046] Dissolve 182g of compound 2 and 135g of phthalic anhydride in 124mL of triethylamine, add 2g of 4-dimethylaminopyridine (DMAP), reflux for 1.5 hours, stop heating, cool to about 50°C and add 40mL of acetic acid Ethyl ester, and then adjust the pH to about 2 with 2N hydrochloric acid, and then stir for half an hour, a large amount of white solid appeared, filtered and washed with water three times to obtain compound 3 with a yield of 93% and a content of 98.5%.
[0047] Alternatives 1-8:
[0048] The preparation method is the same as in Example 2, the difference is that the addition amount of compound 2 and phthalic anhydride is adjusted, and the organic base and the amount thereof are adjusted at the same time to obtain the yield and purity of compound 3 under different reaction conditions as shown in Table 1.
[0049] Table 1,
[0050]
[0051] As shown in table 1: in the preparation process of compound 3, the addition of compound 2 and phthalic anhydride has a gre...
Embodiment 3
[0054] Take 90g (98% content) of compound 3 and heat it to about 40°C and dissolve it in 650mL of absolute ethanol, then add 15g (99% content) of (S)-1-phenylethylamine dropwise, stir for 10 minutes and a large amount of Solid, heated to reflux until all solids disappeared, heated to reflux at 85°C, then slowly cooled to 5°C, kept for 2 hours, filtered, washed with a small amount of ice-water-cooled absolute ethanol to obtain product 4, the yield was 80% , ee% is greater than 99%.
[0055] Replacement Example 9-22:
[0056] The preparation method is the same as in Example 3, the difference is that the solvent, reaction temperature, and reaction time of Example 3 are adjusted, and the synergistic effect between the above-mentioned reaction parameters is tested at the same time. The yield and purity of compound 4 are shown in Table 2.
[0057] Table 2,
[0058]
[0059] As shown in table 2:
[0060] The influence of the choice of solvent on the yield and purity of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com