A kind of method for preparing clorprenaline optical isomers based on chiral liquid chromatography
A technology of optical isomers and clorprenaline, which is applied in organic chemistry methods, chemical instruments and methods, preparation of amino hydroxyl compounds, etc., can solve difficult and unseen problems, and reduce preparation costs and preparation costs Low and fast preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 racemic clorprenaline hydrochloride is raw material
[0030] 1. Sample preparation
[0031] Dissolve 0.1 g of racemic chlorprenaline hydrochloride (Mirrell Chemical Reagent Co., Ltd., Shanghai) in 10 mL of water:methanol=90:10 (volume ratio) mixed solvent, then add 50 μL of triethylamine, swirl for 30 s, pH=8~9.
[0032] 2. Sample separation
[0033] Pre-HPLC adopts dionex ultimate 3000 liquid chromatography system, equipped with WPS-3000SL autosampler and AFC-3000 automatic fraction collection system.
[0034] Chromatographic column: Daicel CHIRALFLASH IF medium pressure preparative column (100mm*30mm*20μm).
[0035] Chromatographic conditions:
[0036] ①The total injection volume is 10mL, set the single injection volume of the autosampler to 2mL, and repeat 5 times;
[0037] 2. eluent is water: the mixed solvent of methanol=90:10 (volume ratio), flow velocity is 12mL / min, isocratic elution;
[0038] ③The temperature of the column thermostat (column ...
Embodiment 2
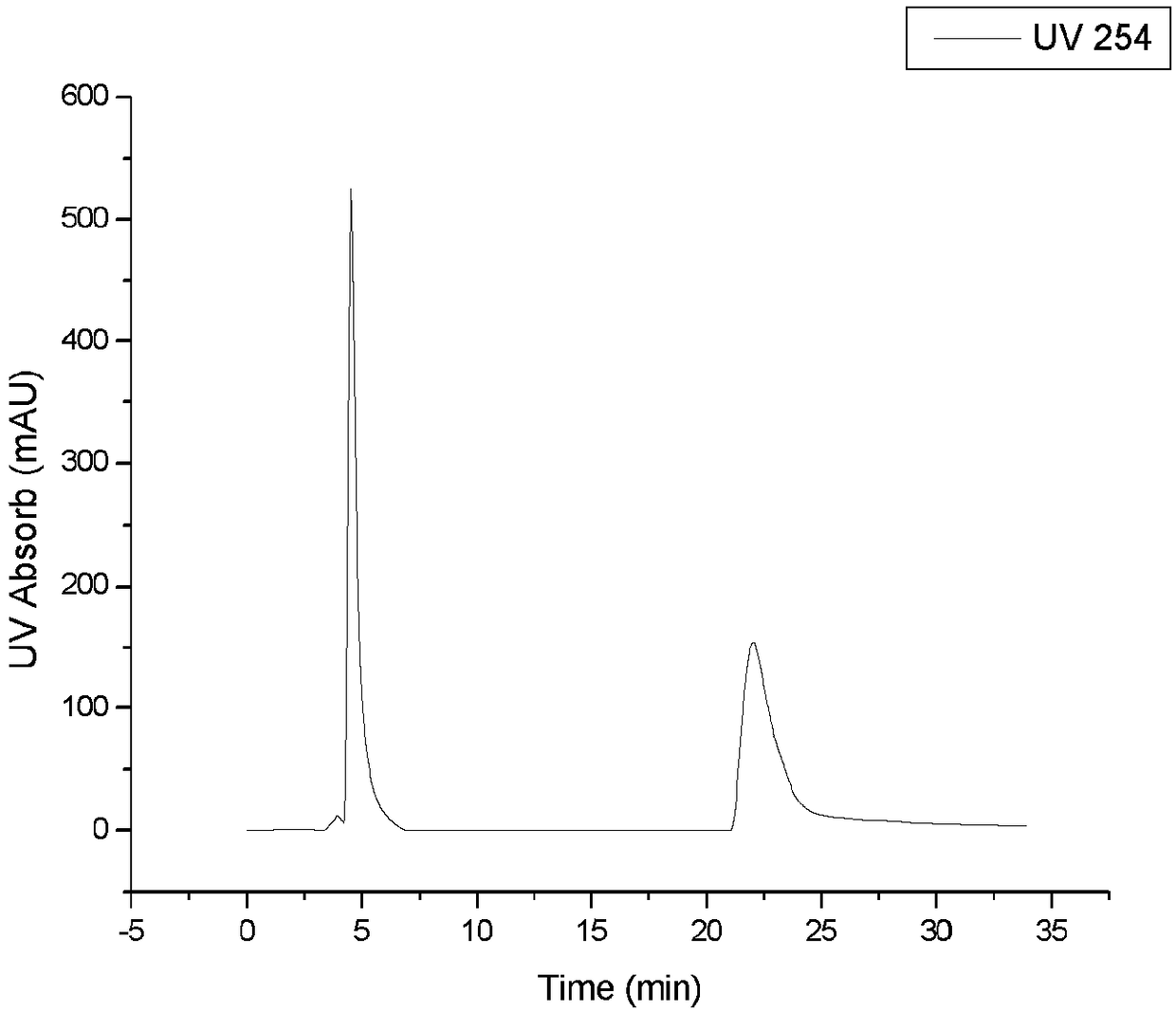
[0057] Embodiment 2 Racemic chlorprenaline is a raw material (seeing for optical purity detection figure 1 )
[0058] Except following conditions, other conditions are identical with embodiment 1:
[0059] 1. 0.1g racemic chlorprenaline (Mai Ruier Chemical Reagent Co., Ltd., Shanghai) was dissolved in 10mL of water: the mixed solvent of methanol=80:20 (volume ratio) (the pH of the obtained injection sample=8~9, no need to adjust );
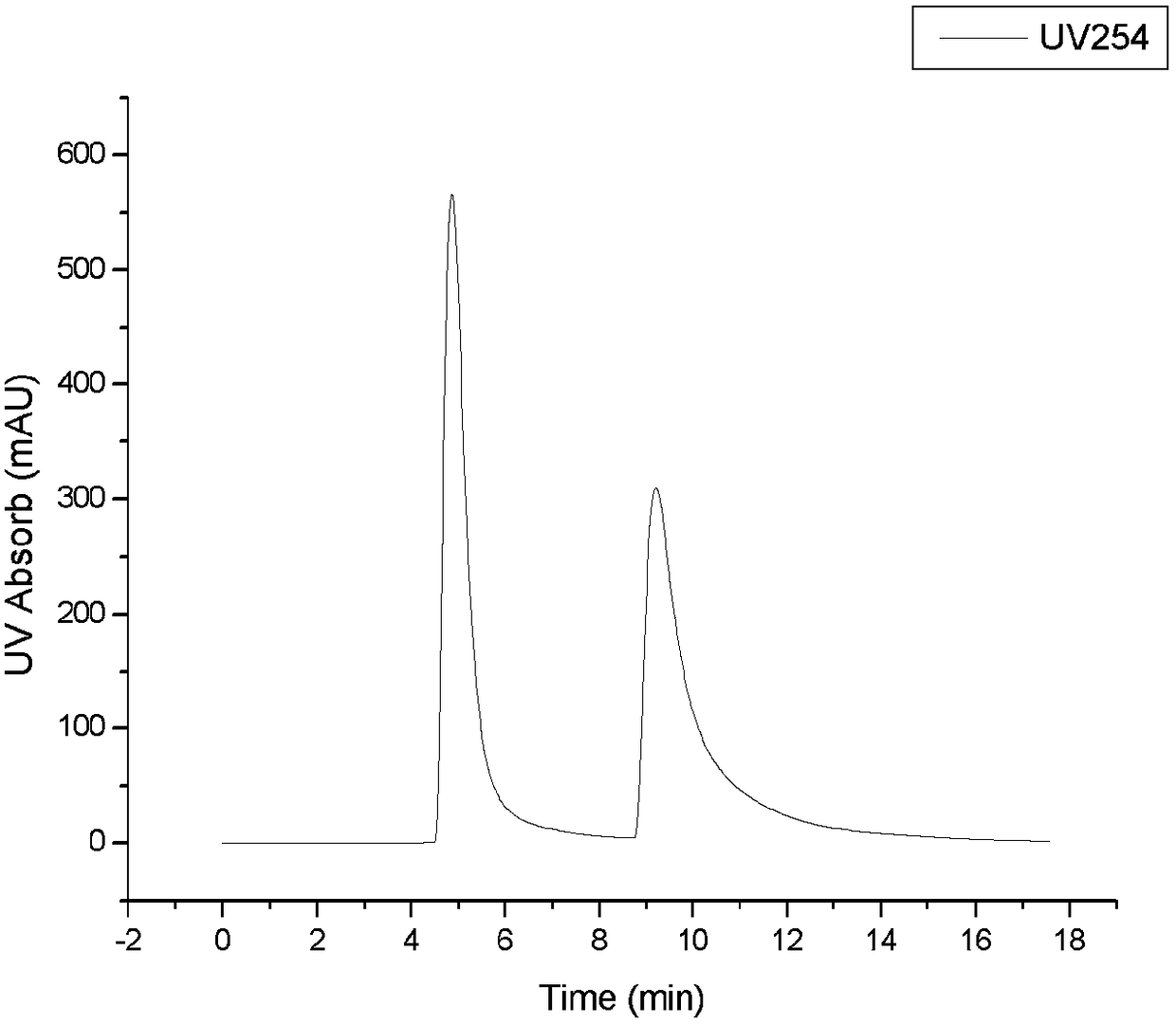
[0060] ②Single injection volume is 2mL (Pre-HPLC results see Figure 2a ). Or the concentration is 40mg / mL (pH=8~9), and the single injection volume is 10mL (Pre-HPLC results see Figure 2b ). The eluent is a mixed solvent of water:ethanol=80:20 (volume ratio), and the flow rate is 12mL / min, isocratic elution.
[0061] ③ The first freeze-drying temperature is -10°C, and the drying time is 48 hours;
[0062] The total recovery rate is 91%, the ordinary purity is greater than 99%, the optical purity of (R)-chlorprenaline is greater than 97%, ...
Embodiment 3
[0063] Embodiment 3 takes the clorprenaline sulfate with low optical purity as raw material
[0064] The raw material sample was analyzed for the purity of the sample in Example 1, and the result was: the ordinary purity was greater than 94%, and the optical purity was 74%.
[0065] Except following conditions, other conditions are identical with embodiment 1:
[0066] ① 0.1 g of the above-mentioned cloprenaline sulfate (Xi'an Pusen Instrument Co., Ltd., Xi'an) was dissolved in a mixed solvent of 15 mL of water:isopropanol=85:15 (volume ratio).
[0067] ② The eluent is a mixed solvent of water:ethanol=85:15 (volume ratio), the flow rate is 15mL / min, isocratic elution.
[0068] ③ The first freeze-drying temperature is -15°C, and the drying time is 24 hours;
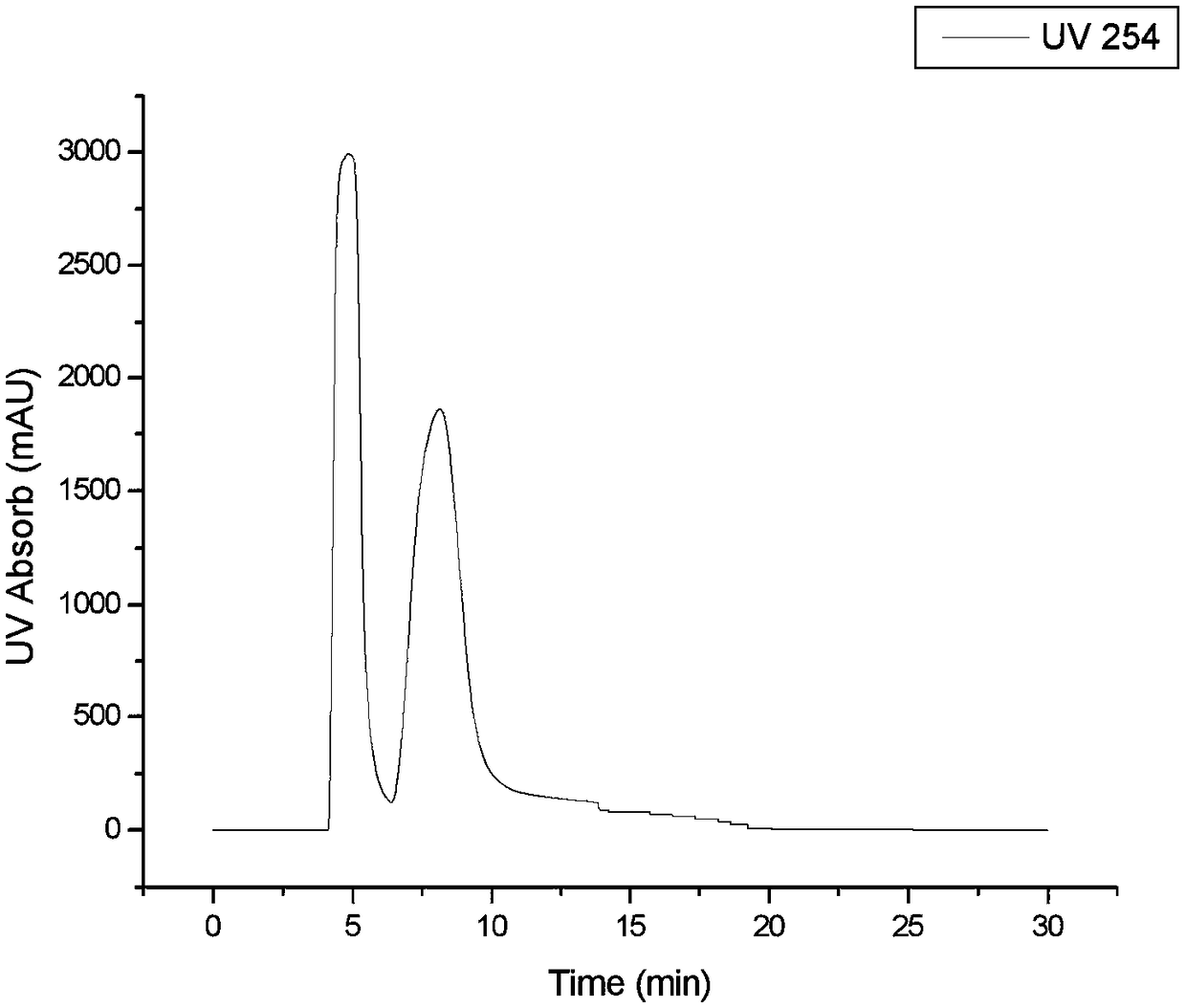
[0069] Total recovery rate is 90%, common purity is all greater than 99%, (R)-chlorprenaline optical purity is greater than 98% ( Figure 3a ), (S)-chlorprenaline optical purity is greater than 97% ( Figure 3b ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com