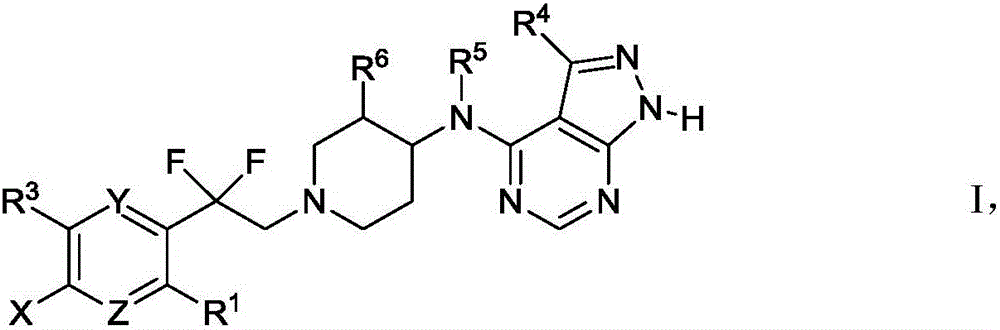
Difluoroethylpyridine derivatives as NR2B NMDA receptor antagonists
A -NO2, alkyl technology, applied in the field of difluoroethylpyridine derivatives as NR2B NMDA receptor antagonists, can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0342] As depicted in the Examples below, in certain exemplary embodiments, chemical entities were prepared according to the following procedures. It should be appreciated that although the general methods illustrate the synthesis of certain chemical entities of the present invention, the following methods and others known to those skilled in the art are applicable to all chemical entities and subclasses and species of each of these chemical entities, as described in this article.
[0343] Temperatures are given in degrees Celsius. If not stated otherwise, all evaporations are performed under reduced pressure, preferably between about 15 mmHg and 100 mmHg. The structures of intermediates and final products were confirmed by standard analytical methods such as mass spectrometry and NMR spectroscopy.
[0344] abbreviation:
[0345] aq water-based
[0346] Boc tert-butoxycarbonyl
[0347] Cbz benzyloxycarbonyl
[0348] DCM dichloromethane
[0349] DCE 1,2-Dichloroethane
...
example 1
[0372] Example 1. Chemical entity.
example 1A
[0373] Example 1.A. Intermediates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com