Methods and compositions related to transplant-associated thrombotic microangiopathy
A technology for microangiopathy and thrombus, applied in the field of evaluation of thrombotic microangiopathy, can solve the problem of lack of screening tools before transplantation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
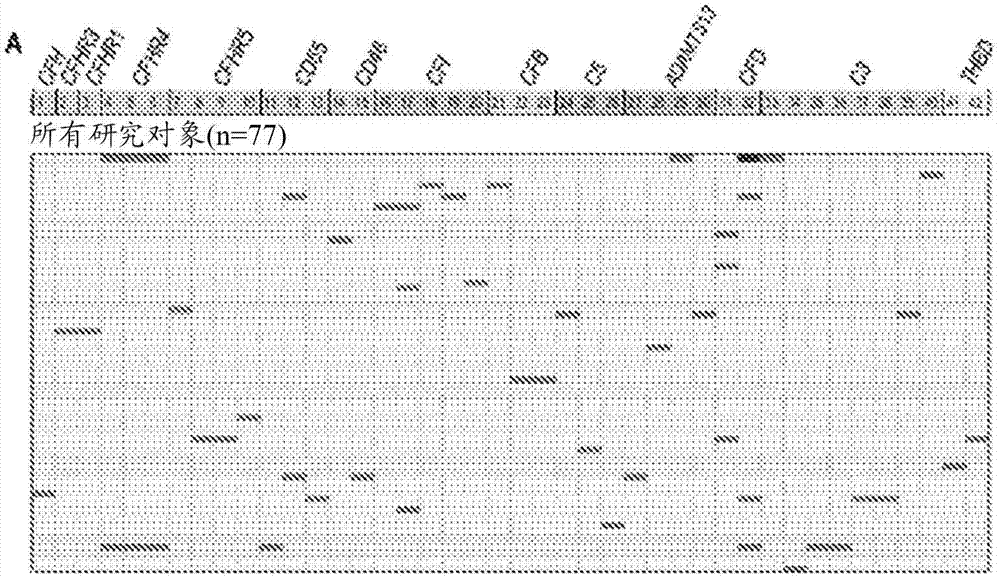
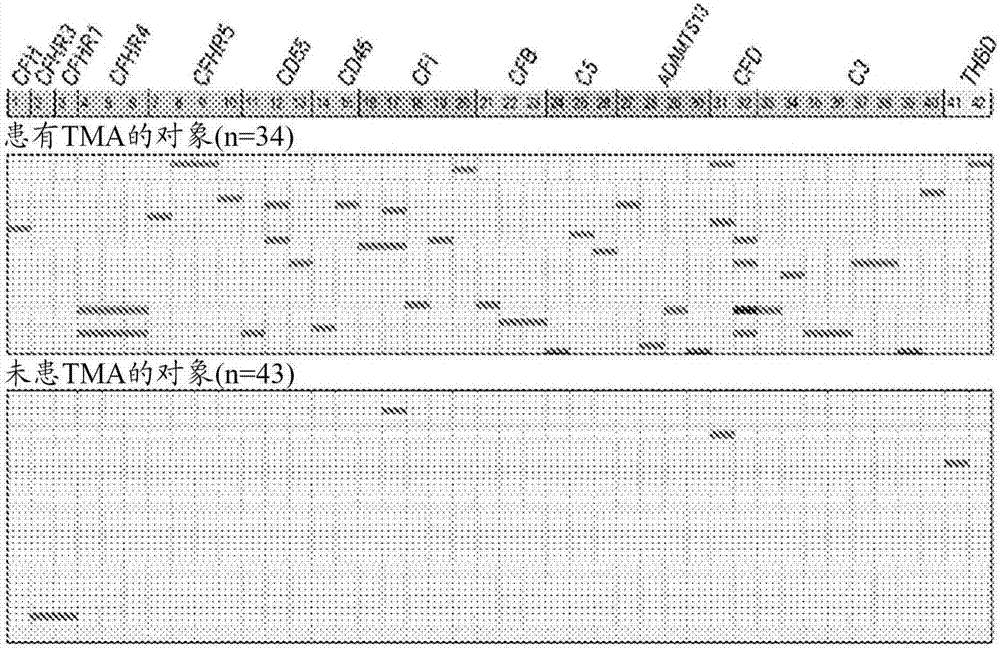
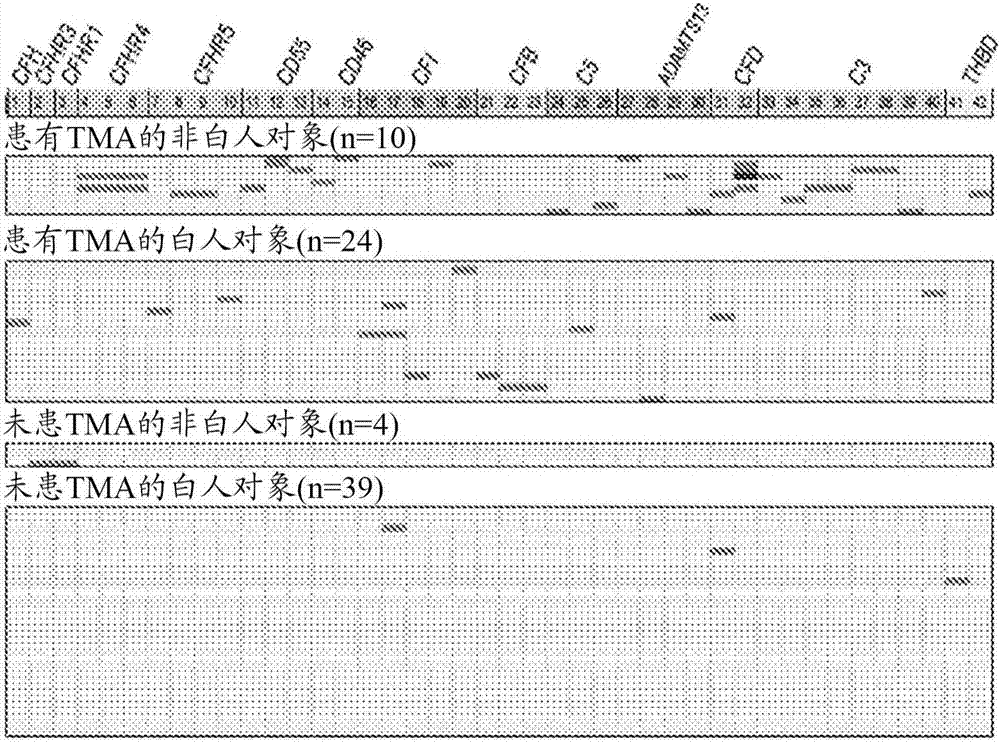
[0069] To further explore the mechanism of TMA, we performed a prospective analysis of 17 candidate genes known to play roles in complement activation. See Jodele S, et al., (2015) Blood Nov 24. pii: blood-2015-08-663435. (Electronic publication prior to press), which is hereby incorporated by reference in its entirety.
[0070] research object
[0071] From September 2010 until December 2011, 100 consecutive patients at Cincinnati Children's Hospital Medical Center (CCHMC) who underwent HSCT were enrolled in the prospective TMA biomarker study after institutional review board approval. Using prospective monitoring as previously described, 39% of subjects met the criteria for TMA (Jodele S, et al. (2014) Blood. 124(4):645-653). Diagnostic criteria for TMA include: (1) lactate dehydrogenase (LDH) above the upper limit of normal; (2) platelet count 9 / L or neothrombocytopenia with ≥50% reduction in platelet count; (3) neonatal anemia with hemoglobin below the lower limit of n...
Embodiment 2
[0098] Eighteen patients showing high-risk features of TA-TMA, including nephrotic-range proteinuria and elevated sC5b-9 levels, were treated with eculizumab (Alexion Pharmaceuticals, Cheshire, CT). Dosing levels were adjusted to maintain a therapeutic eculizumab concentration of 100 μg / ml. See Jodele S. et al., (2015), Biol.Blood MarrowTransplant.pii:S1083-8791(15)00672-2.doi:10.1016 / j.bbmt.2015.10.002. (Electronic publication prior to press), refer It is hereby incorporated by reference in its entirety. Total hemolytic complement activity (CH50) and terminal complement activation (sC5b-9) were observed to monitor complement activation and response to therapy. The results of the treated patients were compared with those of the historical controls from our prospective observational study consisting of 11 individuals who had shown the same high-risk features but were not treated with eculizumab. From the onset of TMA diagnosis, overall survival was compared between the two gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com