A kind of method for adjusting nitrogen content in carbon-nitrogen-based single-atom iron catalyst
An iron catalyst, nitrogen-based single technology, applied in structural parts, electrical components, battery electrodes, etc., can solve the problem of catalyst activity reduction, etc., and achieve the effect of simple and effective preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
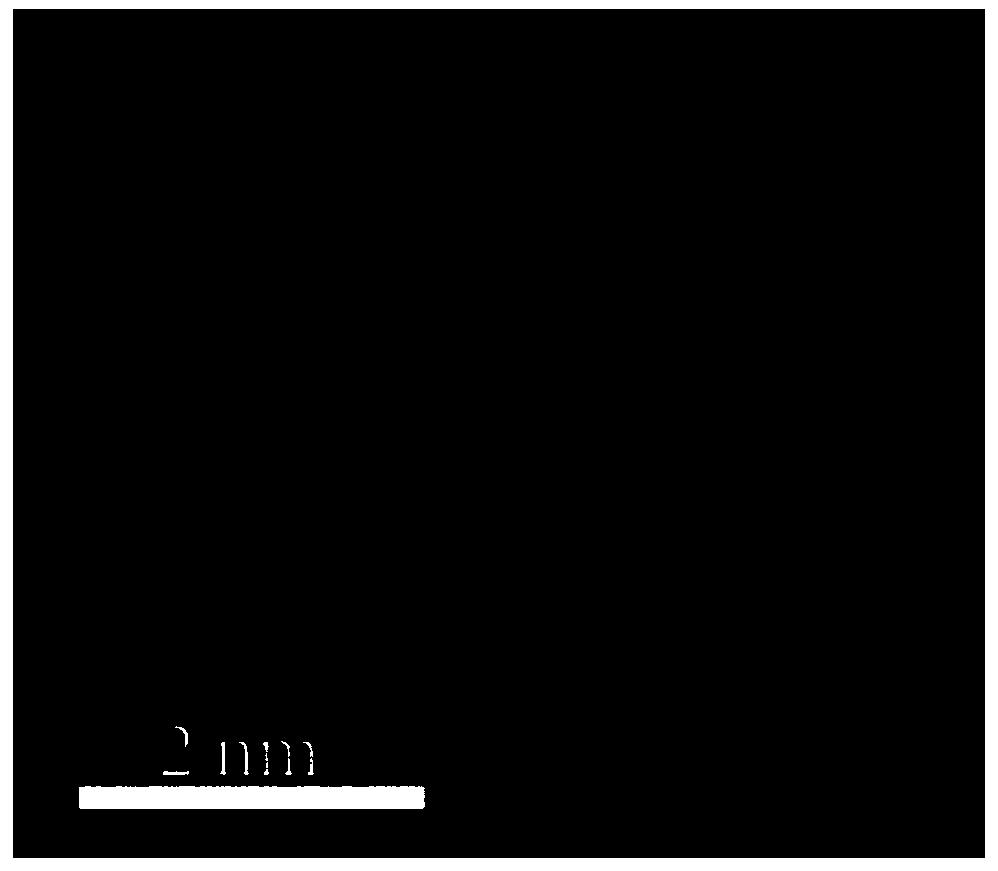
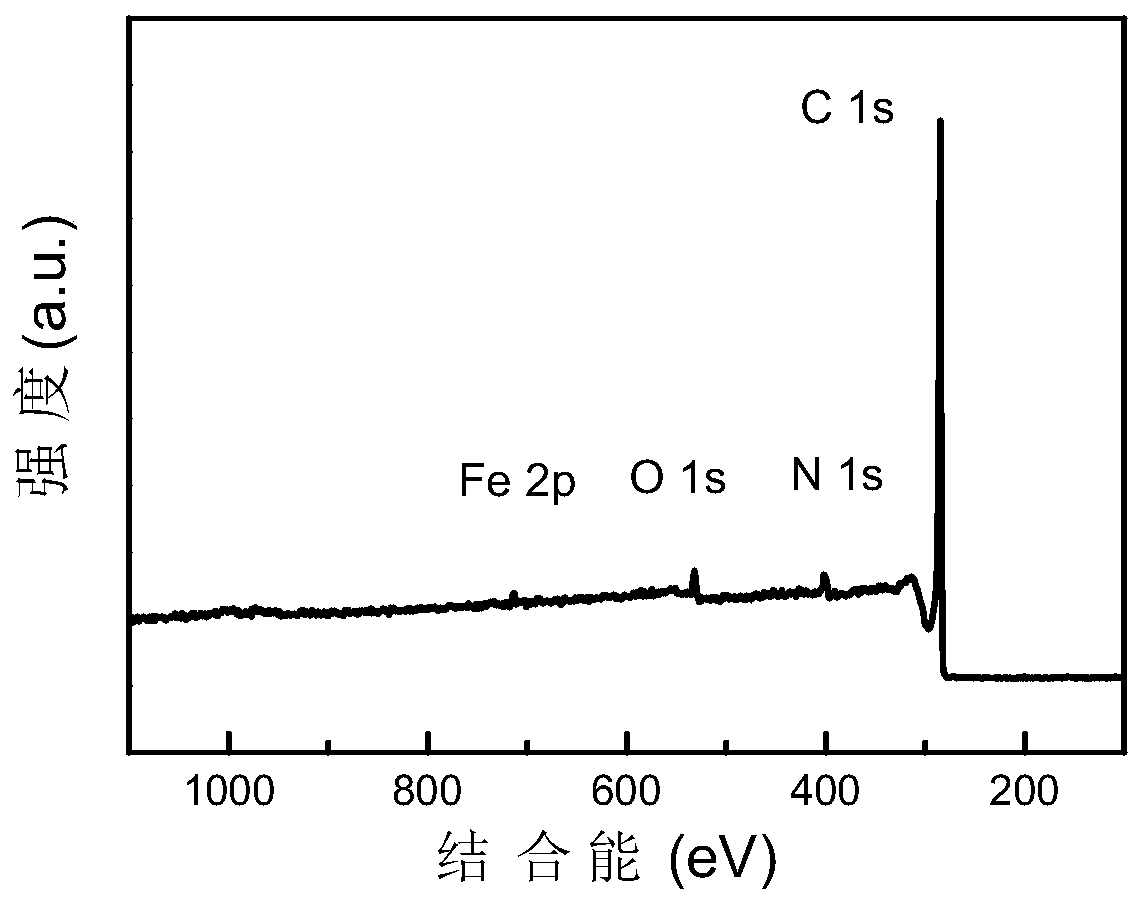
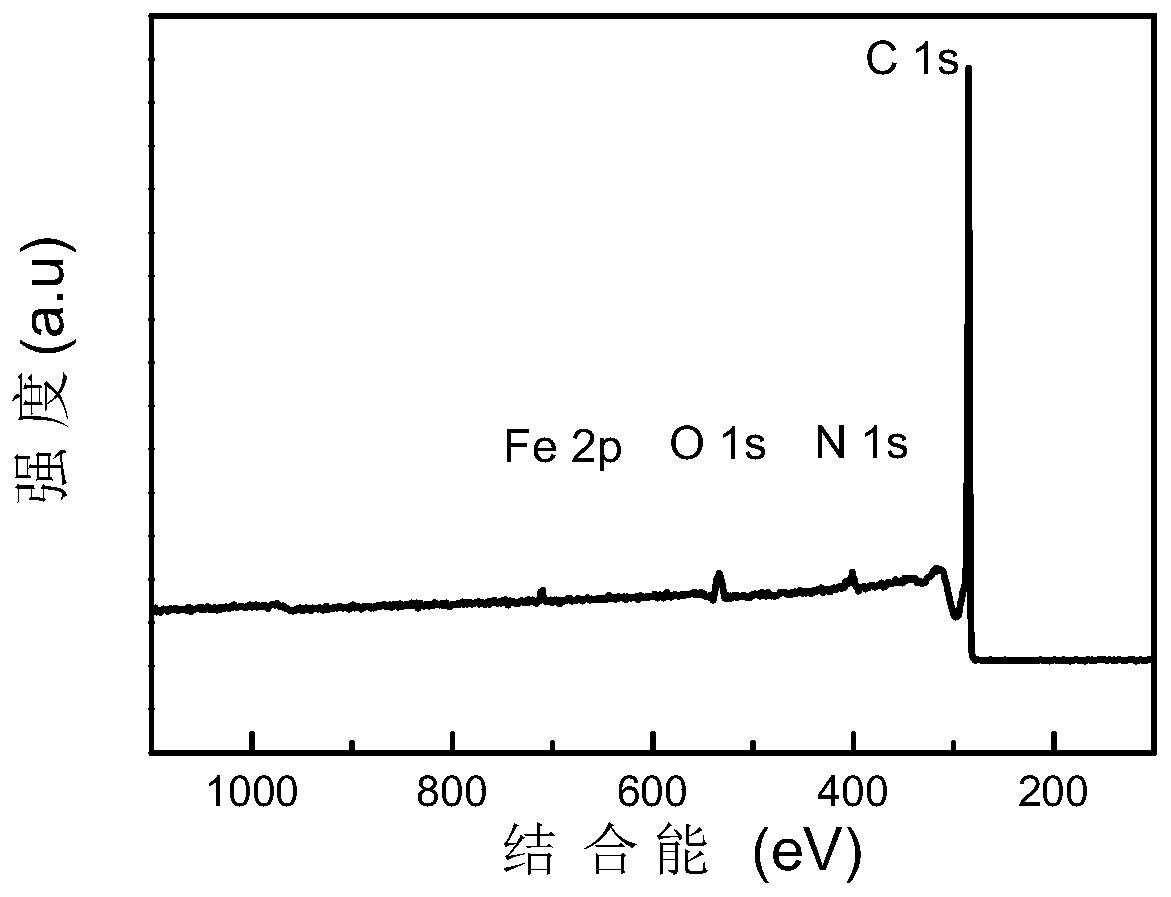
Image
Examples
Embodiment 1
[0038] Dissolve 0.04mol 2-aminopyrimidine in 10mL ethanol (95%) solution to form a transparent solution A; add 0.003mol ferric chloride hexahydrate to the transparent solution A, and stir vigorously at room temperature (20-25°C) for 12 -24h; place the mixture in a vacuum drying oven (80-100°C) and dry (2-4h); place the dried mixture in a tube furnace, and pyrolyze it for 2-4h under a nitrogen atmosphere (heating rate 5°C / min, heated to 1000°C), cooled to room temperature to obtain a black solid; use sulfuric acid or hydrochloric acid (2-6mol / L, 80mL) to acidify the obtained black solid at 80-100°C for 12-24h to remove the material Medium-active metal iron and its oxides; then centrifuge and wash the acid-washed material (until the pH value of the lotion reaches 7) and dry (8-12h) in a vacuum drying oven (80-100°C); The dried product is subjected to secondary high-temperature pyrolysis for 2-4h (the temperature rise rate is 5°C / min, and the temperature is raised to 1000°C), and...
Embodiment 2
[0040]Dissolve 0.04mol N-methylimidazole in 10mL ethanol (95%) solution to form a transparent solution A; add 0.003mol ferric chloride hexahydrate to the transparent solution A, and stir vigorously at room temperature (20-25°C) 12-24h; place the mixture in a vacuum drying oven (80-100°C) and dry (2-4h); place the dried mixture in a tube furnace, and pyrolyze it for 2-4h under a nitrogen atmosphere (heating The rate is 5°C / min, the temperature is raised to 1000°C), cooled to room temperature to obtain a black solid; acidify the obtained black solid with sulfuric acid or hydrochloric acid (2-6mol / L, 80mL) at 80-100°C for 12-24h to remove The active metal iron and its oxides in the material; then the acid-washed material is centrifuged, washed (until the pH value of the washing liquid reaches 7) and dried in a vacuum drying oven (80-100°C) (8-12h) The dried product is subjected to secondary high-temperature pyrolysis for 2-4h (the heating rate is 5° C. / min, and the temperature is...
Embodiment 3
[0042] Dissolve 0.04mol 2-benzimidazolyl acetonitrile in 10mL ethanol (95%) solution to form a transparent solution A; Stir vigorously for 12-24h; place the mixture in a vacuum oven (80-100°C) and dry (2-4h); place the dried mixture in a tube furnace, and pyrolyze it for 2-4h under a nitrogen atmosphere (The heating rate is 5°C / min, the temperature is raised to 1000°C), cooled to room temperature to obtain a black solid; use sulfuric acid or hydrochloric acid (2-6mol / L, 80mL) to acidify the obtained black solid at 80-100°C for 12-24h To remove the active metal iron and its oxides in the material; then centrifuge and wash the acid-washed material (until the pH value of the washing liquid reaches 7) and dry it in a vacuum drying oven (80-100°C) (8- 12h); the dried product is subjected to secondary high-temperature pyrolysis for 2-4h (heating rate is 5°C / min, and the temperature is raised to 1000°C), cooled to room temperature, and a Fe-N-C single-atom catalyst with a nitrogen co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com