Method for adjusting nitrogen content in carbon-nitrogen based monatomic ferrous catalyst
An iron catalyst, nitrogen-based single technology, applied in electrical components, battery electrodes, circuits, etc., can solve problems such as catalyst activity reduction, and achieve the effect of simple and effective preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
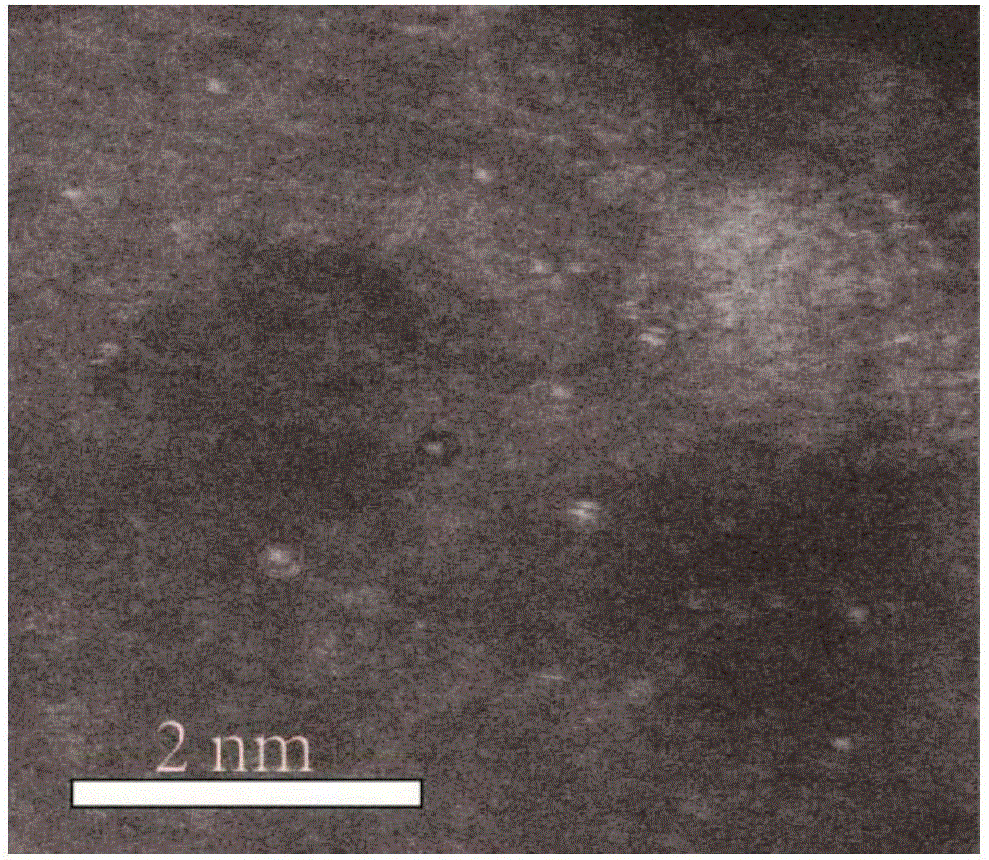
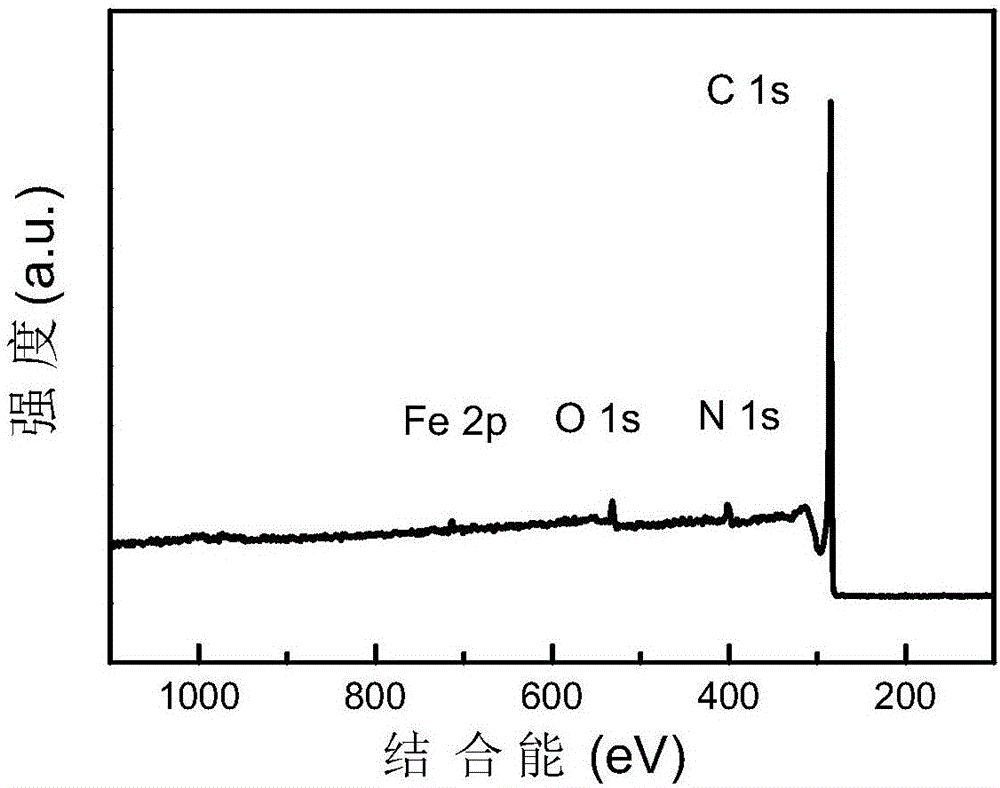
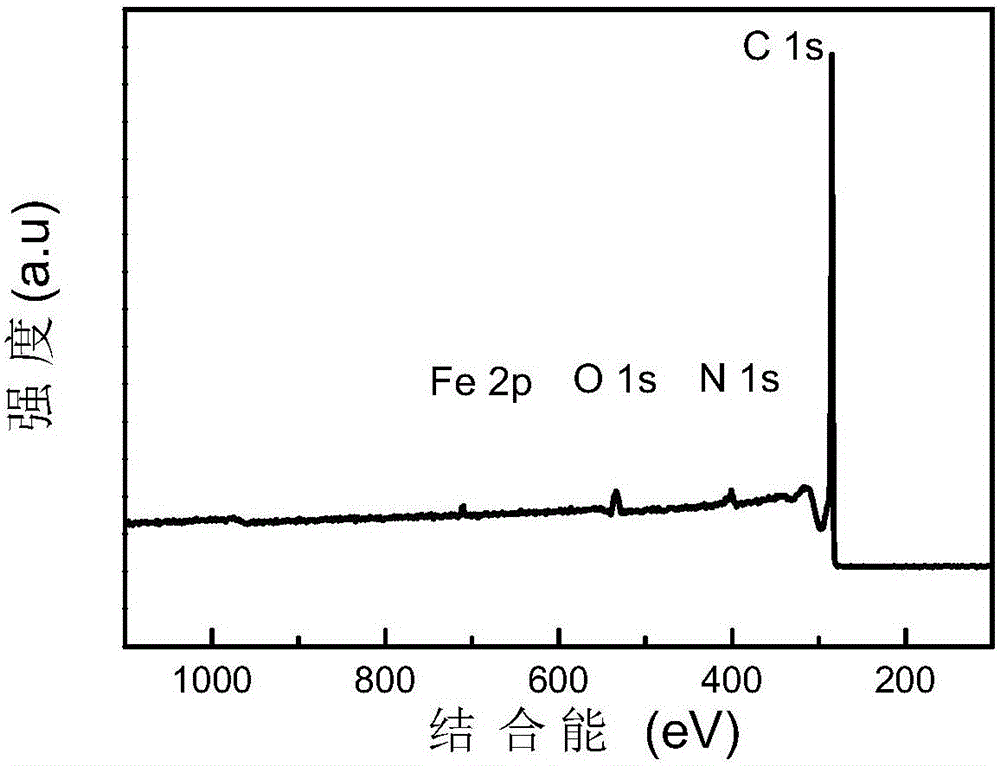
[0038] Dissolve 0.04mol 2-aminopyrimidine in 10mL ethanol (95%) solution to form a transparent solution A; add 0.003mol ferric chloride hexahydrate to the transparent solution A, and stir vigorously at room temperature (20-25°C) for 12 -24h; place the mixture in a vacuum drying oven (80-100°C) and dry (2-4h); place the dried mixture in a tube furnace, and pyrolyze it for 2-4h under a nitrogen atmosphere (heating rate 5°C / min, heated to 1000°C), cooled to room temperature to obtain a black solid; use sulfuric acid or hydrochloric acid (2-6mol / L, 80mL) to acidify the obtained black solid at 80-100°C for 12-24h to remove the material Medium-active metal iron and its oxides; then centrifuge and wash the acid-washed material (until the pH value of the lotion reaches 7) and dry (8-12h) in a vacuum drying oven (80-100°C); The dried product is subjected to secondary high-temperature pyrolysis for 2-4h (the temperature rise rate is 5°C / min, and the temperature is raised to 1000°C), and...
Embodiment 2
[0040]Dissolve 0.04mol N-methylimidazole in 10mL ethanol (95%) solution to form a transparent solution A; add 0.003mol ferric chloride hexahydrate to the transparent solution A, and stir vigorously at room temperature (20-25°C) 12-24h; place the mixture in a vacuum drying oven (80-100°C) and dry (2-4h); place the dried mixture in a tube furnace, and pyrolyze it for 2-4h under a nitrogen atmosphere (heating The rate is 5°C / min, the temperature is raised to 1000°C), cooled to room temperature to obtain a black solid; acidify the obtained black solid with sulfuric acid or hydrochloric acid (2-6mol / L, 80mL) at 80-100°C for 12-24h to remove The active metal iron and its oxides in the material; then the acid-washed material is centrifuged, washed (until the pH value of the washing liquid reaches 7) and dried in a vacuum drying oven (80-100°C) (8-12h) The dried product is subjected to secondary high-temperature pyrolysis for 2-4h (the heating rate is 5° C. / min, and the temperature is...
Embodiment 3
[0042] Dissolve 0.04mol 2-benzimidazolyl acetonitrile in 10mL ethanol (95%) solution to form a transparent solution A; Stir vigorously for 12-24h; place the mixture in a vacuum oven (80-100°C) and dry (2-4h); place the dried mixture in a tube furnace, and pyrolyze it for 2-4h under a nitrogen atmosphere (The heating rate is 5°C / min, the temperature is raised to 1000°C), cooled to room temperature to obtain a black solid; use sulfuric acid or hydrochloric acid (2-6mol / L, 80mL) to acidify the obtained black solid at 80-100°C for 12-24h To remove the active metal iron and its oxides in the material; then centrifuge and wash the acid-washed material (until the pH value of the washing liquid reaches 7) and dry it in a vacuum drying oven (80-100°C) (8- 12h); the dried product is subjected to secondary high-temperature pyrolysis for 2-4h (heating rate is 5°C / min, and the temperature is raised to 1000°C), cooled to room temperature, and a Fe-N-C single-atom catalyst with a nitrogen co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com