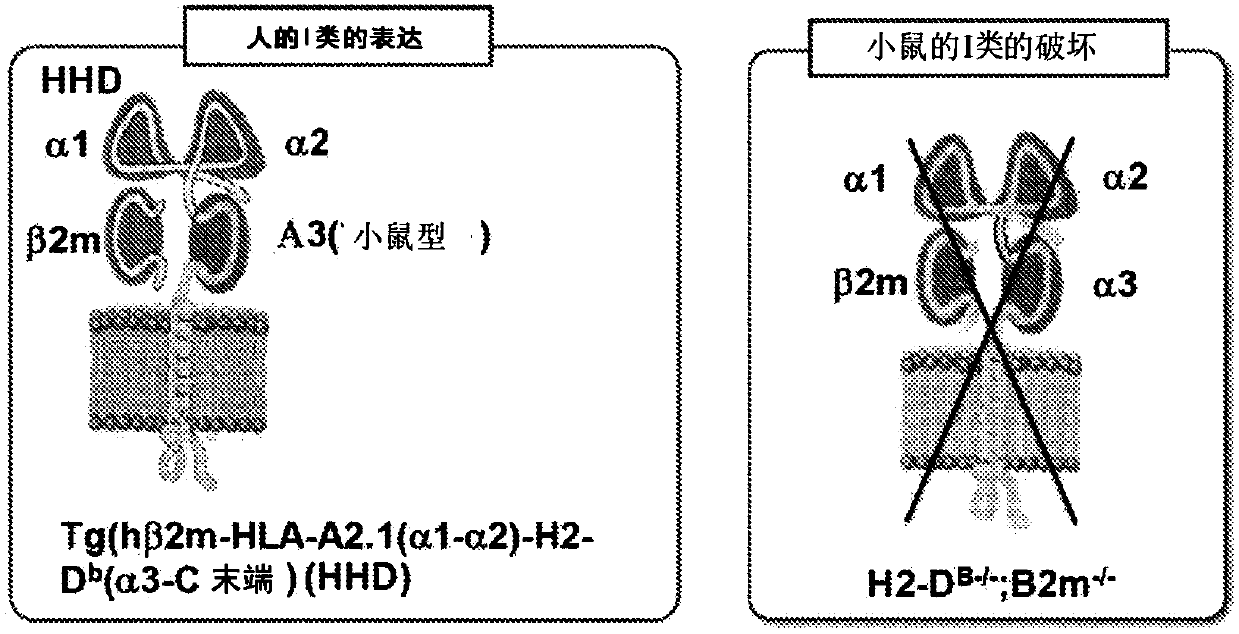
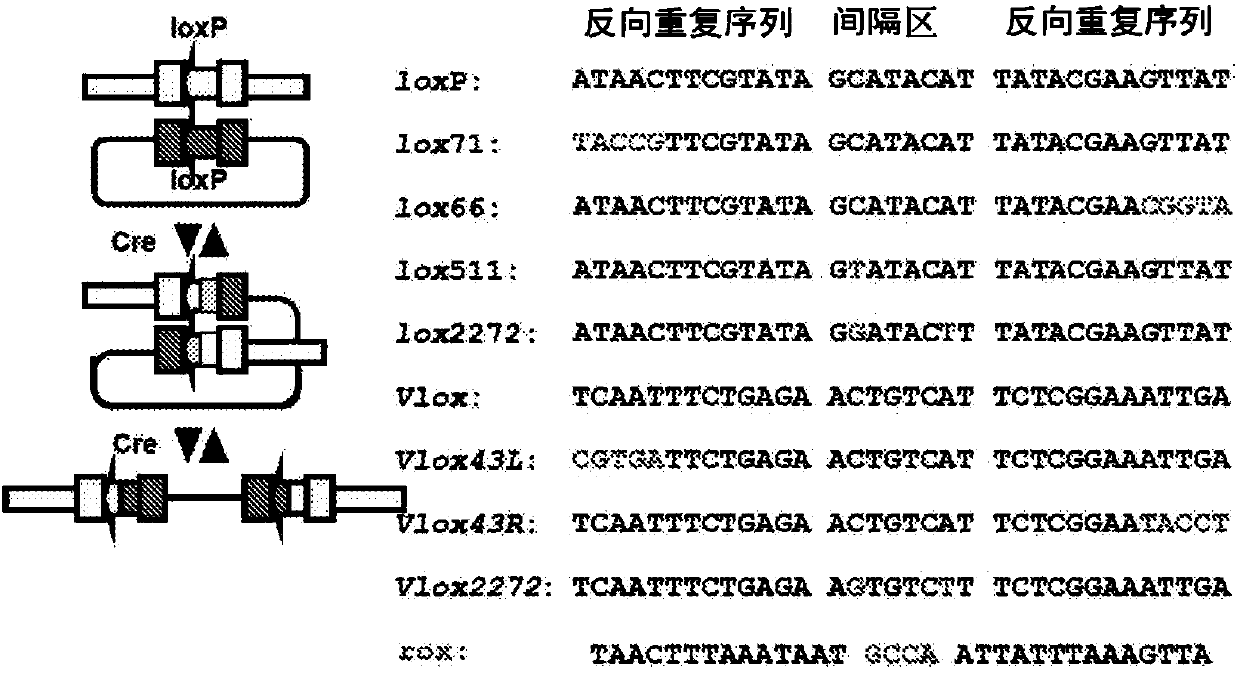
Organ-humanized mouse
A mouse and human technology, applied in biochemical equipment and methods, artificially induced pluripotent cells, embryonic cells, etc., can solve problems such as difficulty in obtaining multiple mice and difficulty in breeding NOG mice.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0232] Establishment of ES cells
[0233] In this example, in order to establish the most suitable humanized mice for human hepatocyte transplantation, ES cell lines were established from HHB mouse embryos, and mouse strains were also established.
[0234] (1) Establishment of HHB mice and establishment of ES cell lines
[0235] HHB mice were used for in vitro fertilization to obtain 33 blastocyst embryos. CHIR99021, an inhibitor of GSK3, and PD0325901, an inhibitor of MEK, which are effective for maintaining the undifferentiated state of ES, were added to the medium (GMEM- KSR-2i medium), try to establish ES.
[0236] Specifically, embryos of HHB were collected by in vitro fertilization. 33 blastocysts were cultured on KSOM medium for 4 days until embryo sacs formed, and 1 embryo was placed in each of 48 wells (coated with gelatin only). The medium used was KSR-GMEM-2i medium. As the medium composition, G-MEM (Glasgow minimum essential medium) contains 1X MEM non-essentia...
Embodiment 2
[0244] Induction of mouse hepatocyte death
[0245] (1) Production of a construct for inducing mouse hepatocyte death
[0246] In order to produce genetically modified mice capable of specific disappearance of hepatocytes, two constructs were produced.
[0247] Construct 1 (CAG-ATG-lox-EGFP-lox-DT-A) had ATG, lox-clamped EGFP, and DT-A (diphtheria toxin fragment A) linked immediately downstream of the CAG promoter.
[0248] The start codon of EGFP and the ATG upstream of ROX were designed in frame fit. Additionally, the start codon for DT-A was removed and designed in such a way that it fits in the ATG framework upstream of rox.
[0249] Construct 2 (SAP-CreER T2) is linked with Cre-ER immediately downstream of the promoter of hepatocyte-specific serum amyloid P component (SAP) T2 . Furthermore, a puromycin resistance gene was linked upstream of the SAP promoter.
[0250] The specific method is as follows.
[0251] (1-1) Construct 1
[0252] Construct 1 was made as fol...
Embodiment 3
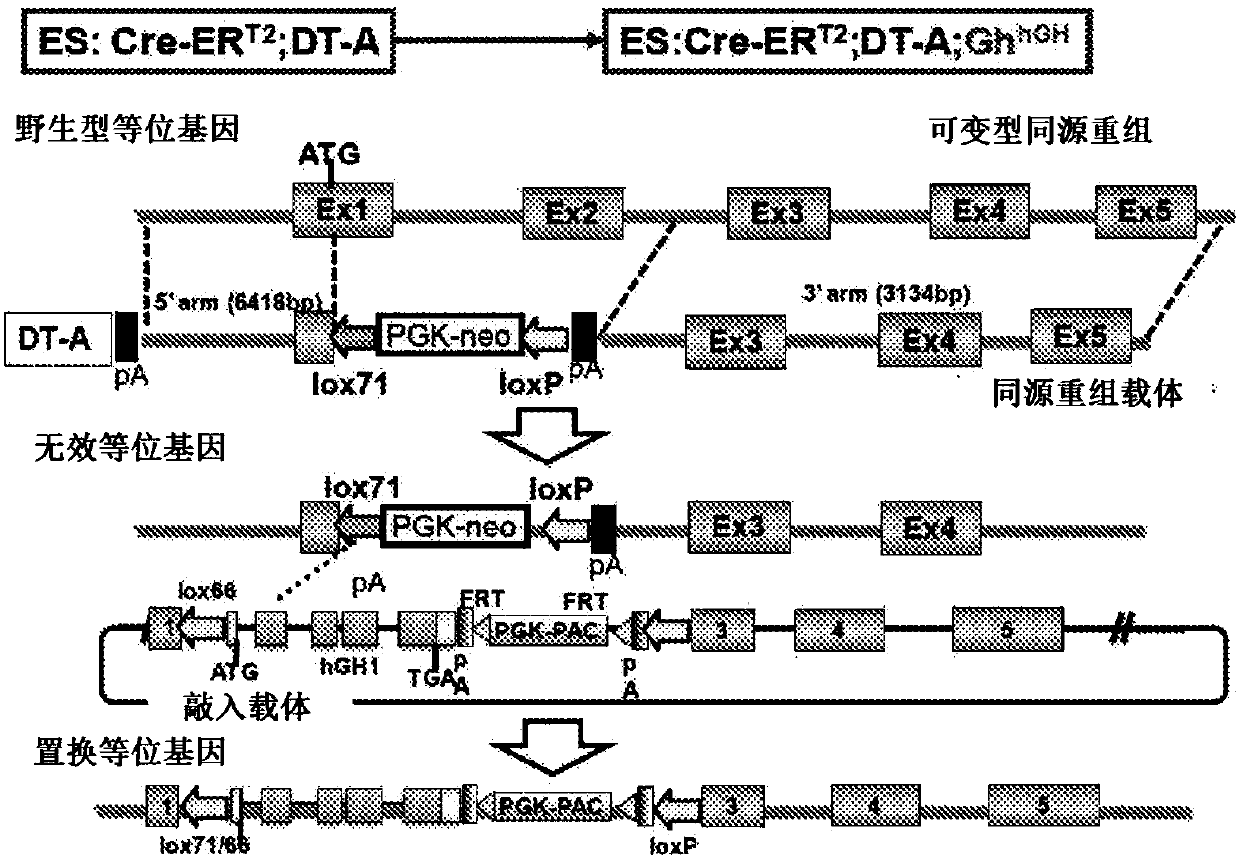
[0280] Human Growth Hormone Gene Replacement
[0281] Using a homologous recombination vector, exon 1 and exon 2 of the mouse growth hormone (Gh) gene were pre-destructed in the same manner as in Example 2 by conventional methods. At this time, the ATG of the first exon was destroyed, and the target recombinant clone in which lox71-PGK-beta-geo-loxP-poly A-lox2272 was embedded was obtained. Then make the replacement vector. The replacement vector contains lox66-genomic hGH gene-polyA-Frt-PGK-puro-Frt-loxP. This replacement vector was introduced into the target recombinant clone by electroporation together with the Cre expression vector.
[0282] As a result, an ES clone in which the mouse Gh gene was replaced with the human GH gene was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com