Benzodiazepine compounds
A technology for benzodiazepines and compounds is applied in the field of benzodiazepine compounds and their preparation, and can solve the problems such as separation of degraded impurities, structure confirmation, unstable compounds, and low content that have not yet been seen.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of compound (R is methyl) shown in embodiment 1 formula (I)
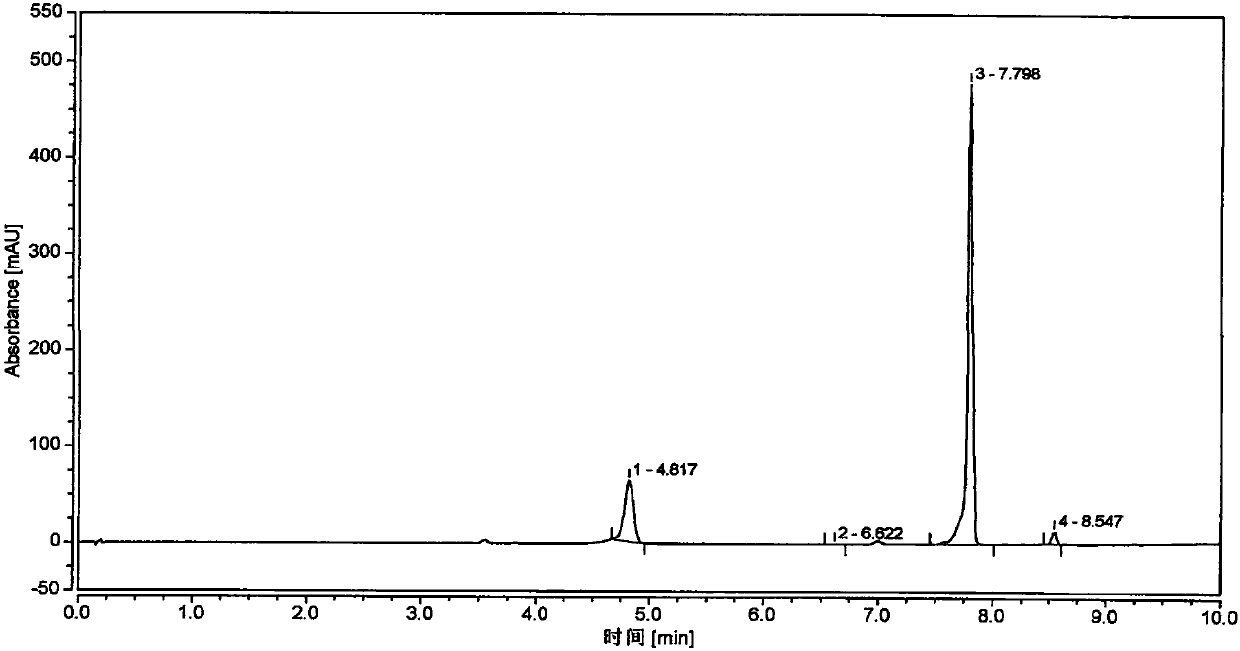
[0065] Dissolve 5 g of the compound represented by formula (II) (R is methyl) in 50 ml of dichloromethane, add 7 g of DBN, stir at 40°C until the reaction is complete, wash with acetic acid aqueous solution to pH 8-9, and dry overnight with anhydrous magnesium sulfate , concentrated, and passed through the column (eluent is ethyl acetate:petroleum ether=1:30), the eluent was concentrated under reduced pressure, the residue was heated to 60°C with a small amount of ethanol, 1g of oxalic acid was added, stirred for 30min, and cooled to 0~ Stir and crystallize at 5°C, filter, and neutralize the filter cake with sodium bicarbonate, and form a salt with benzenesulfonic acid to obtain 0.5 g of the compound represented by formula (I). The content is 98.5% as measured by HPLC area normalization method.
[0066] Mp: 195.3℃~197.4℃
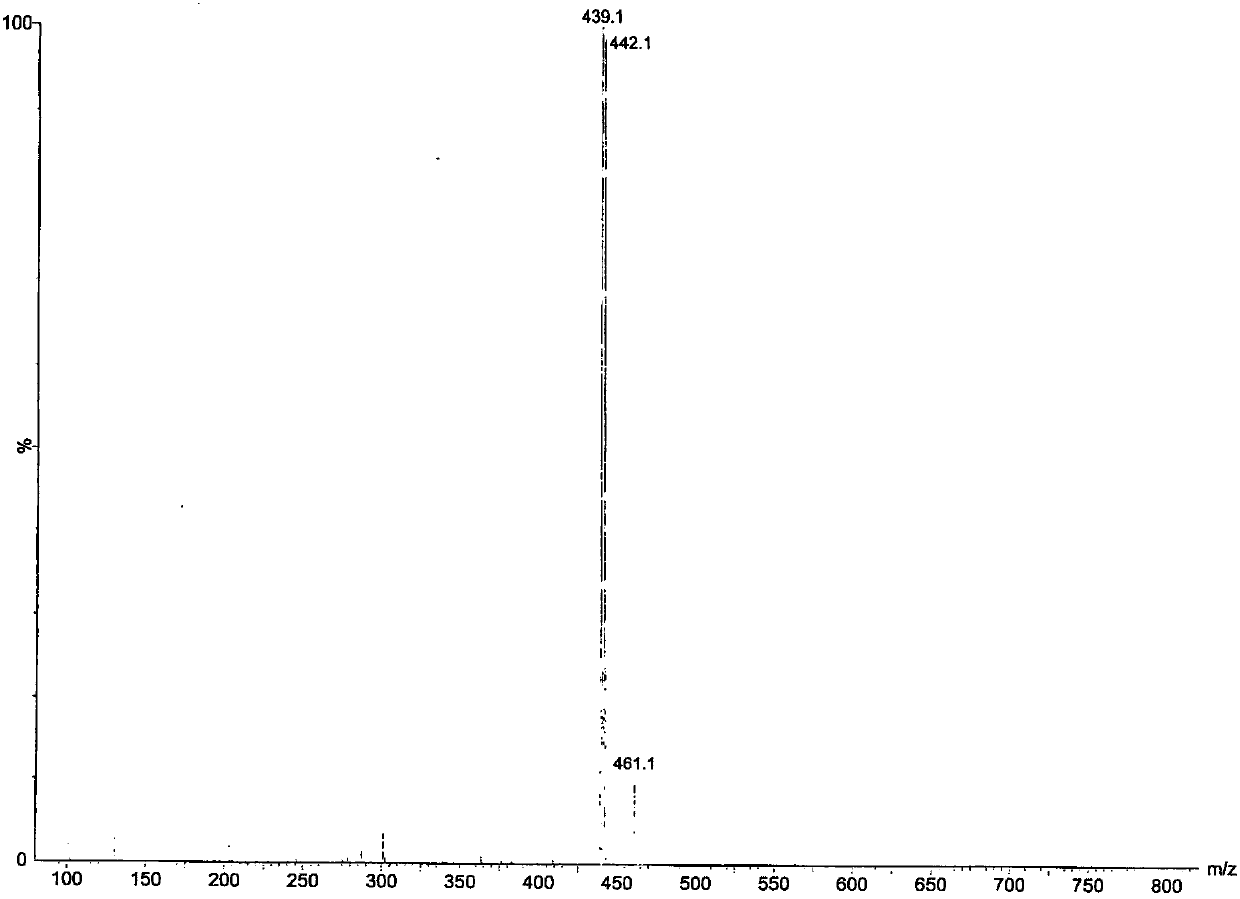
[0067] MS: 439.1[M+H] + , 461.1[M+Na] +
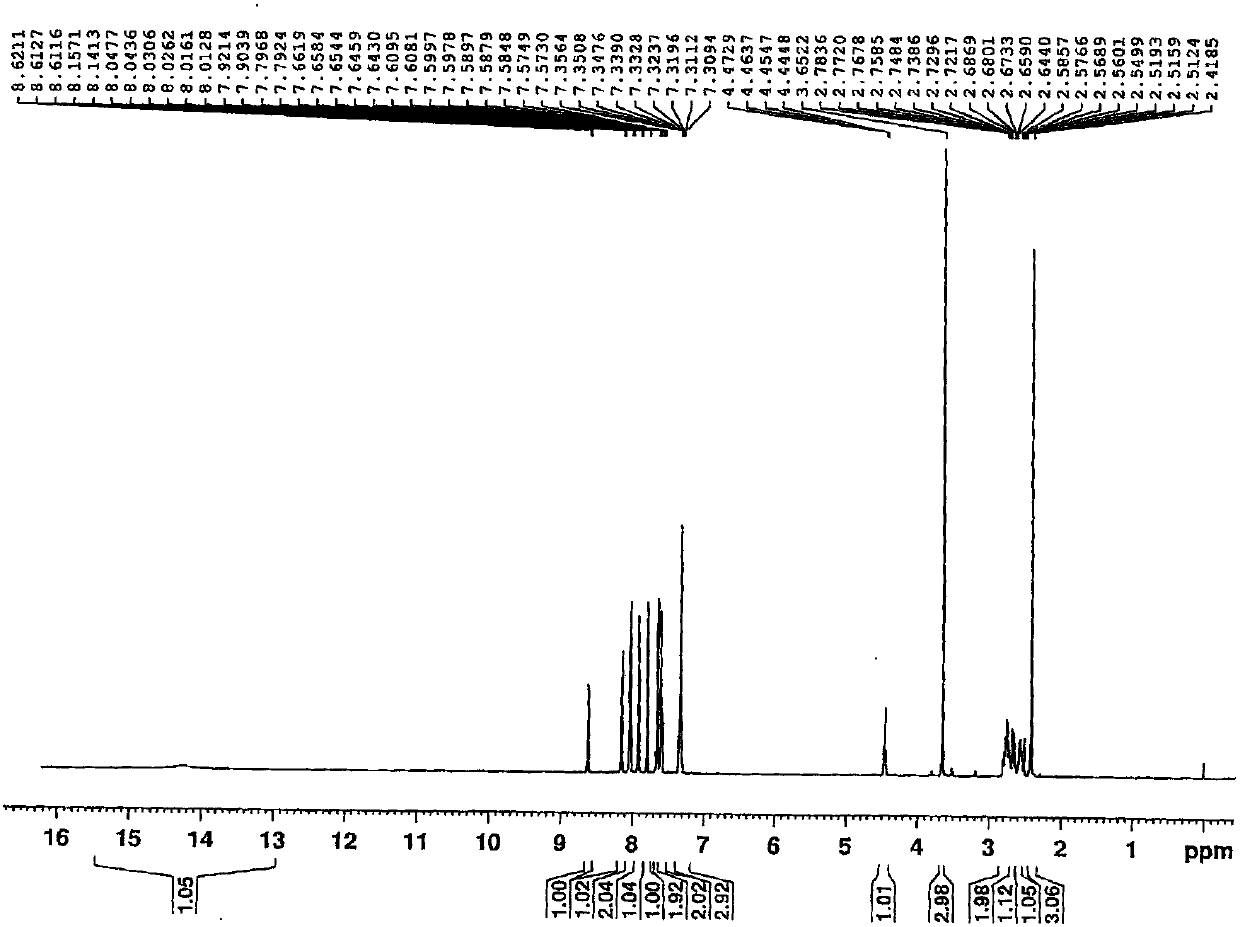
[0068] 1 H-NMR (500MHz, ...
Embodiment 2
[0070] The preparation of compound (R is hydrogen) shown in embodiment 2 formula (I)
[0071] Dissolve 5 g of the compound represented by formula (II) (R is hydrogen) in 35 ml of acetonitrile, add 10 g of DBU, stir at 70°C until the reaction is complete, add 100 ml of dichloromethane, wash with acetic acid aqueous solution to pH 8-9, anhydrous sulfuric acid Dry the magnesium overnight, concentrate, pass through the column (eluent is ethyl acetate:petroleum ether=1:30), concentrate the eluent under reduced pressure, heat the residue to 45°C with a small amount of acetone, add 1g of oxalic acid, stir for 30min, cool down Stir and crystallize at 0-5°C, filter, neutralize the filter cake with sodium bicarbonate, and form a salt with benzenesulfonic acid to obtain 0.35 g of the compound represented by formula (I).
[0072] Mp: 183.9℃~185.3℃
[0073] MS: 425.1[M+H] +
Embodiment 3
[0074] The preparation of compound (R is ethyl) shown in embodiment 3 formula (I)
[0075] Dissolve 5 g of the compound represented by formula (II) (R is ethyl) in 60 ml of tetrahydrofuran, add 12 g of TBD, stir at 60°C until the reaction is complete, add 100 ml of dichloromethane, wash with acetic acid aqueous solution to pH 8-9, anhydrous Magnesium sulfate was dried overnight, concentrated, and passed through the column (eluent: ethyl acetate: petroleum ether = 1:30), the eluent was concentrated under reduced pressure, and the residue was separated with a preparative liquid phase. The mobile phase was methanol: acetonitrile: Water: triethylamine = 45: 25: 30: 0.01, the fractions were received and concentrated, and salted with benzenesulfonic acid to obtain 0.65 g of the compound represented by formula (I).
[0076] Mp: 202.3℃~204.1℃
[0077] MS: 453.3 [M+H] +
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap