A method and system for evaluating the reliability of toxicological data
A technology of reliability and toxicology, applied in the field of evaluation methods and systems for the reliability of toxicological data, can solve the problems of lack of quantitative and practical evaluation tools, and achieve the effect of good external authenticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
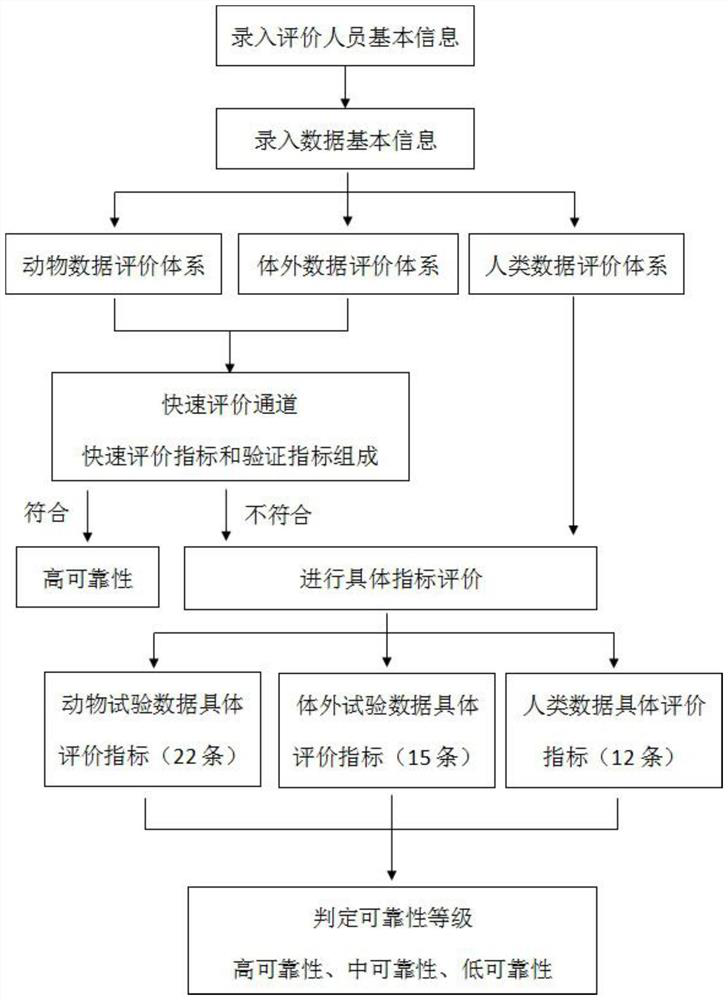
[0036] For animal test data, a rapid evaluation channel has been established. After the evaluation indicators of the rapid evaluation channel are met and confirmed by further verification, it can be directly evaluated as a high reliability level, otherwise specific index evaluation is required.
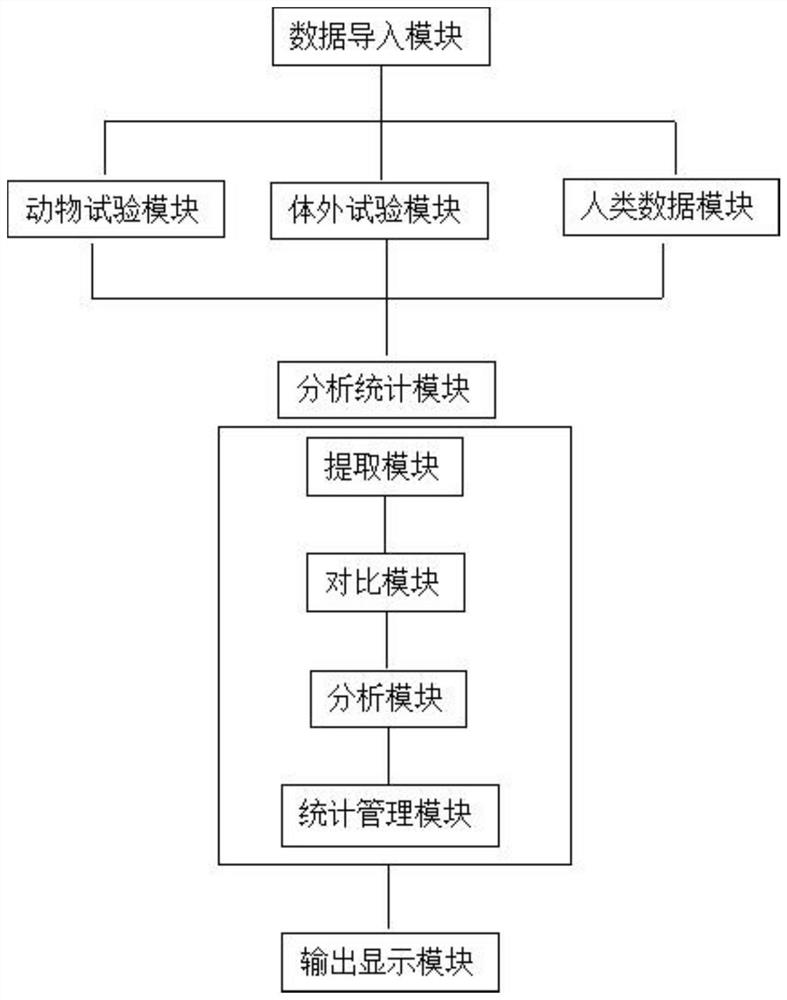
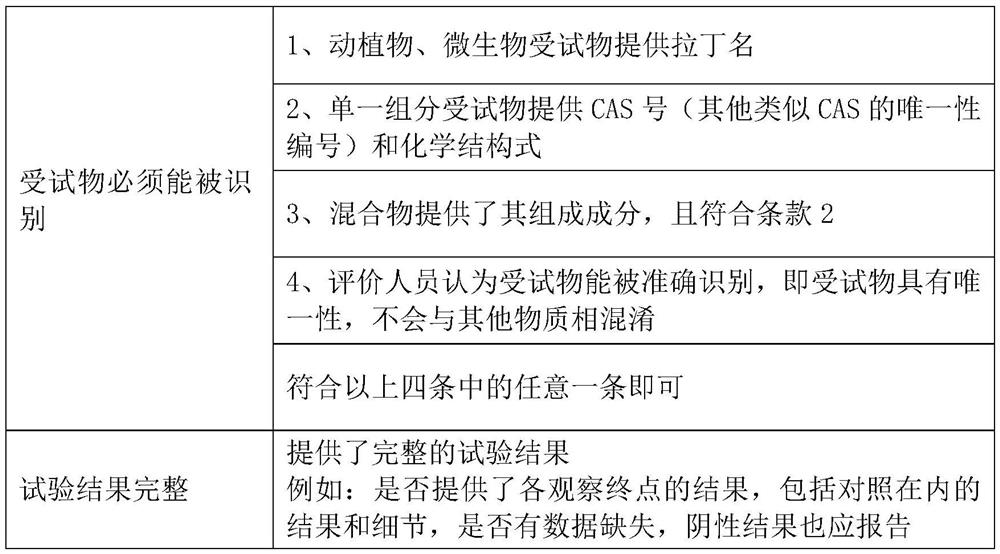
[0037] For the data that has not passed the rapid evaluation channel, the reliability evaluation is carried out from five parts: test substance, experimental animal, experimental design, experimental operation and method, experimental result and conclusion. It includes 22 specific evaluation indicators. Comparing with the scoring elements and suggested scores listed in the evaluation indicators, score items one by one according to whether the scoring elements are satisfied, multiply by the weight corresponding to each evaluation indicator, and calculate the percentage (actual score / total score ), the reliability of the data is divided into three levels of high, medium and low reliabili...
Embodiment 2
[0074] For in vitro test data, a rapid evaluation channel has been established. After the evaluation indicators of the rapid evaluation channel are met and confirmed by further verification, it can be directly evaluated as a high reliability level, otherwise specific index evaluation is required.
[0075] For data that has not passed the rapid evaluation channel, the reliability evaluation is carried out from five parts: test substance, test system, test design, test operation and method, test result and conclusion. It includes 15 specific evaluation indicators. Comparing with the scoring elements and suggested scores listed in the evaluation indicators, score each item according to whether the scoring elements are satisfied, multiply by the weight corresponding to each evaluation indicator, and calculate the percentage. Percentage = actual score / The total score divides the reliability of the data into three levels of high, medium and low reliability according to the percenta...
Embodiment 3
[0095] For human data, the reliability evaluation is carried out from five parts: research type and research object, measurement, quality control, bias control, results and conclusion. It includes 12 specific evaluation indicators. Comparing with the scoring elements and suggested scores listed in the evaluation indicators, according to whether the scoring elements are satisfied or not, the scores are scored one by one, multiplied by the weight corresponding to each evaluation indicator, and the percentage is calculated. Percentage = actual score / The total score divides the reliability of the data into three levels of high, medium and low reliability according to the percentage.
[0096] For example, for a human data.
[0097] First, enter the basic information such as the name, title, and work unit of the evaluator; and enter the basic information such as the author's name, title, publication year, and author's institution for the toxicological data.
[0098] Scores are ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com