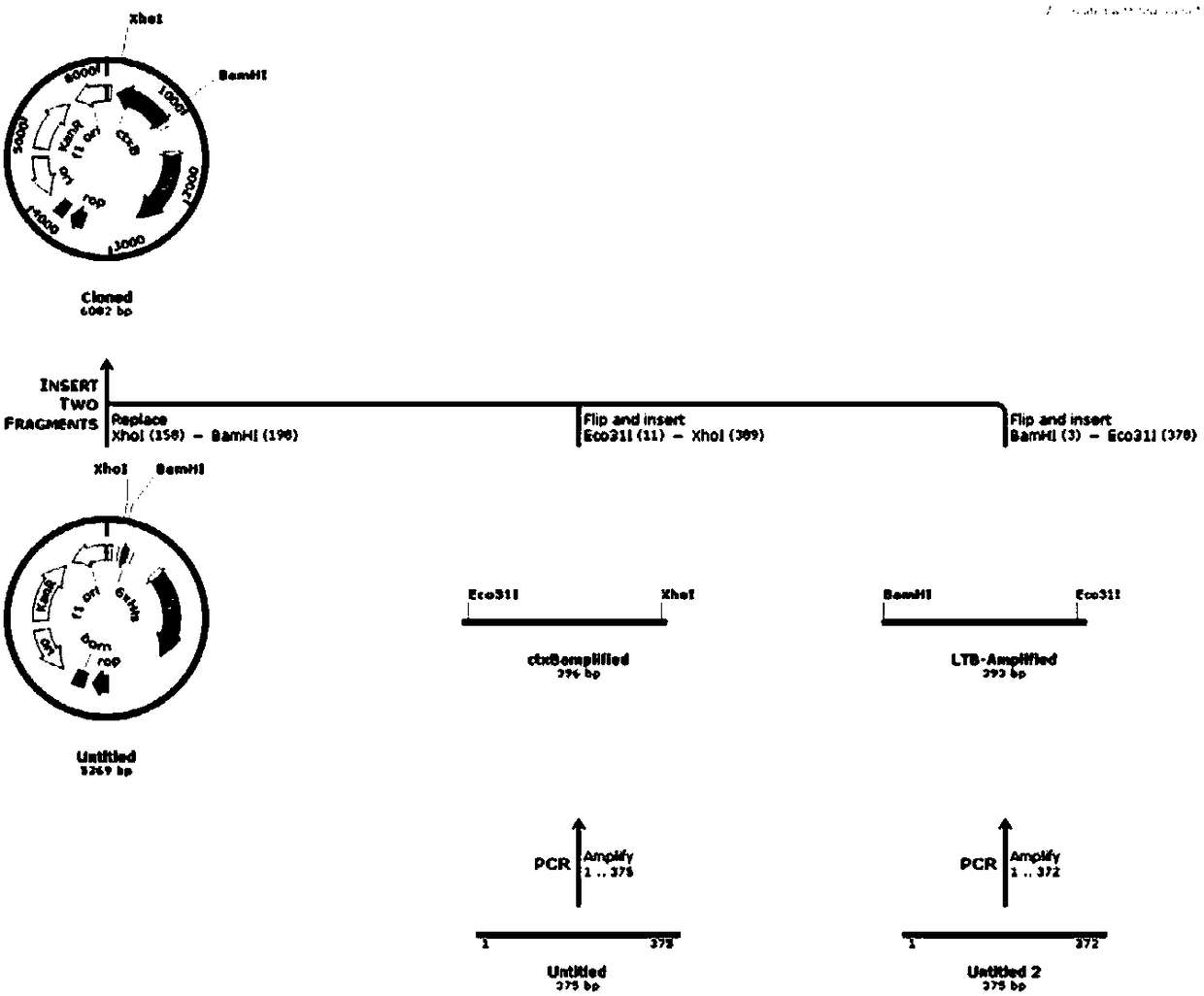
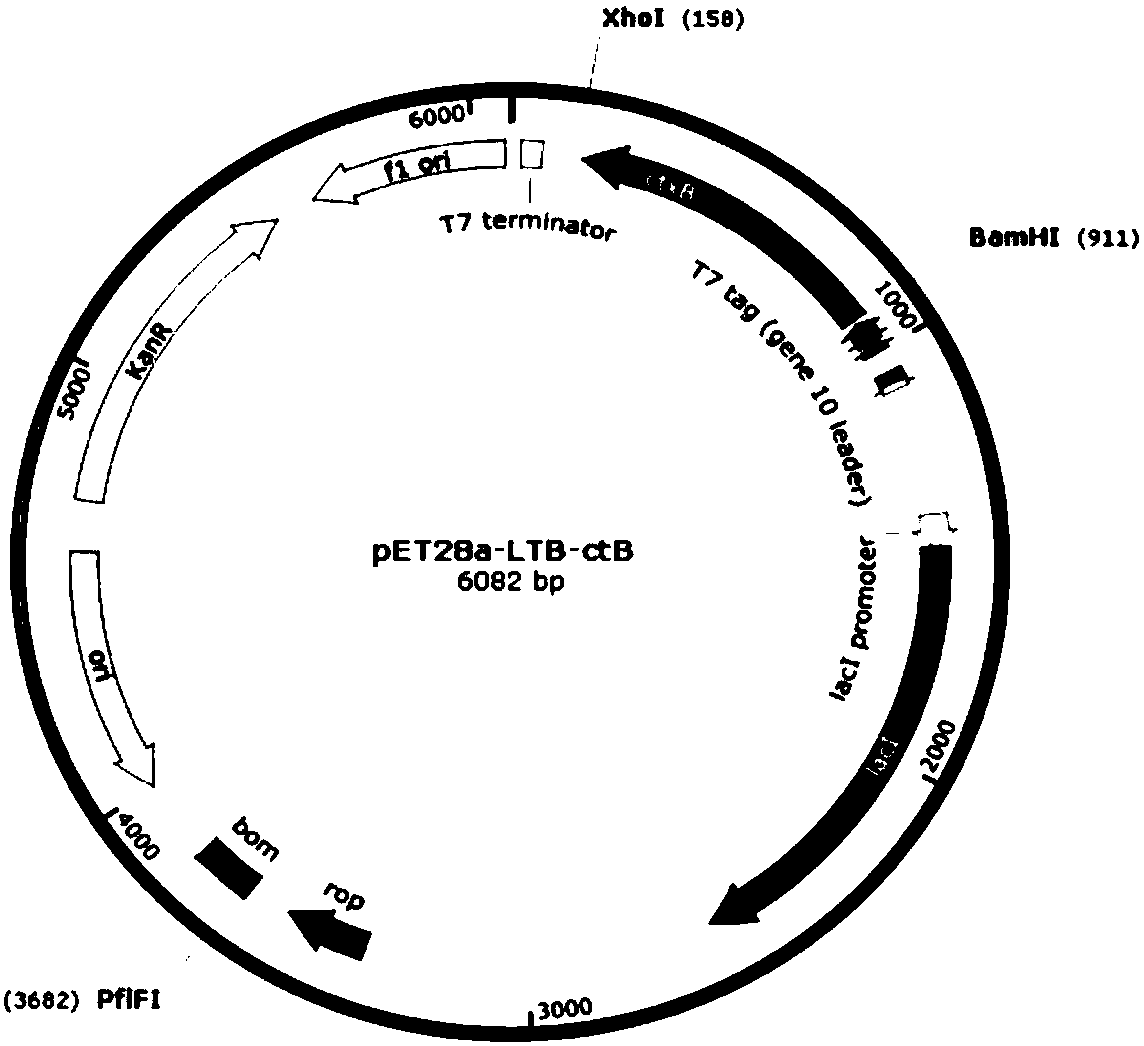
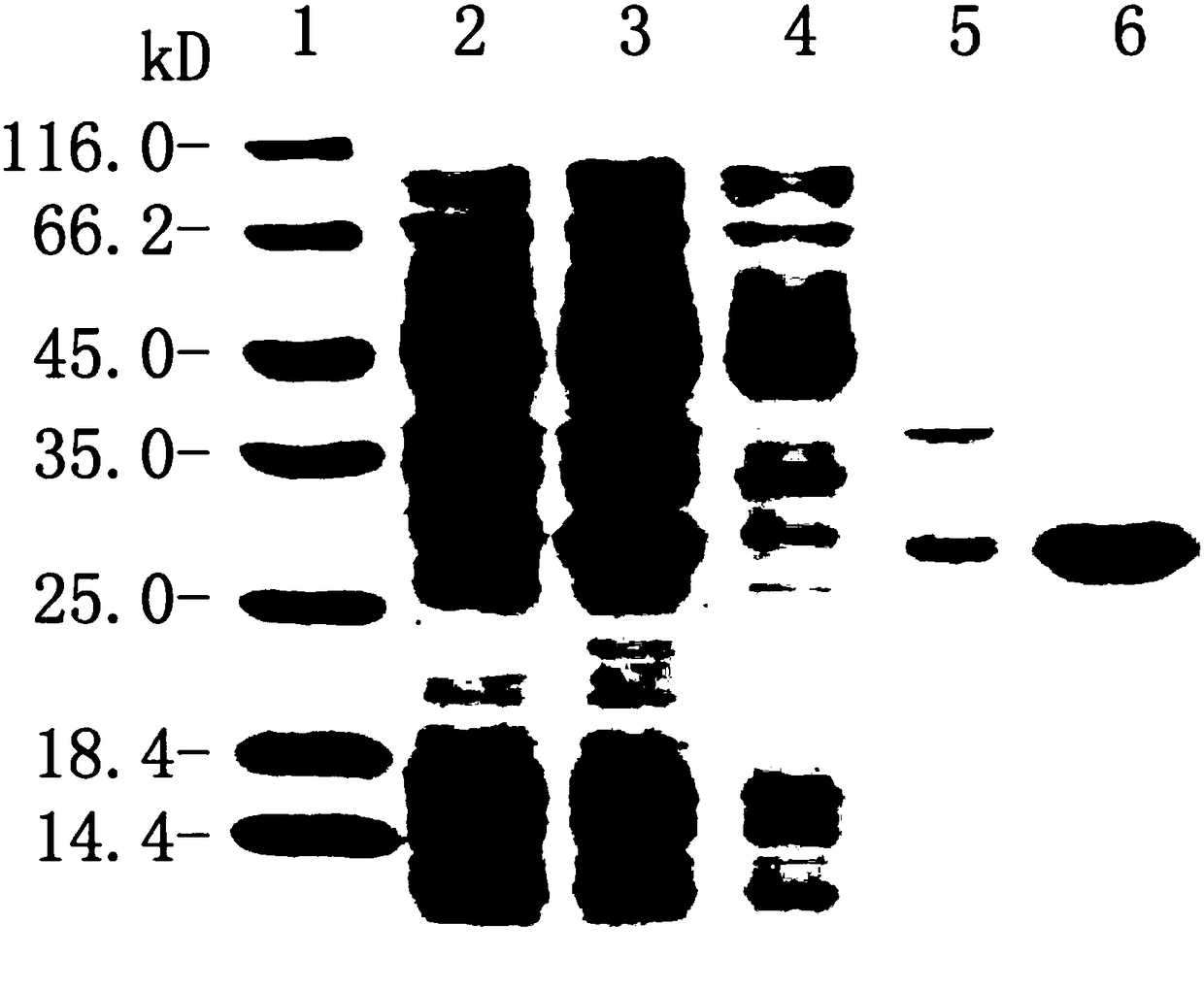
Pneumococcus combined vaccine using recombinant vector protein and preparation method of pneumococcus combined vaccine
A technology of pneumococcus and recombinant vector, applied in the biological field, can solve the problems of destroying immune effect, difficult vaccine co-existence, lack of cross-immune protection effect, etc., to achieve the effect of enhancing immune response and wide use value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Cloning and prokaryotic expression of recombinant LTB and CTB proteins
[0062] LT is the same as CT and so on. LT is an AB5 hexameric protein composed of A and B subunits (LT-A and LT-B). The two subunits A and B are combined by non-covalent bonds, and the individual subunits have no biological activity, and only when they are combined together can they have the biological and chemical characteristics of a full toxin. The A subunit is the virulence center of the toxin with a molecular weight of about 28KD and has ADP-ribosylase activity. The A subunit can be cut into two fragments, A1 (LT-A1) and A2 (LT-A2), by reduction reaction. The A1 fragment and the A2 fragment respectively have a folded structure and a helical structure, and the two are connected by a disulfide bond. A1 is the active part of the LT toxin, and A2 is the part linked to the B subunit. When the disulfide bond connecting A1 and A2 is reduced, the enzymatically active A1 subunit is released. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com