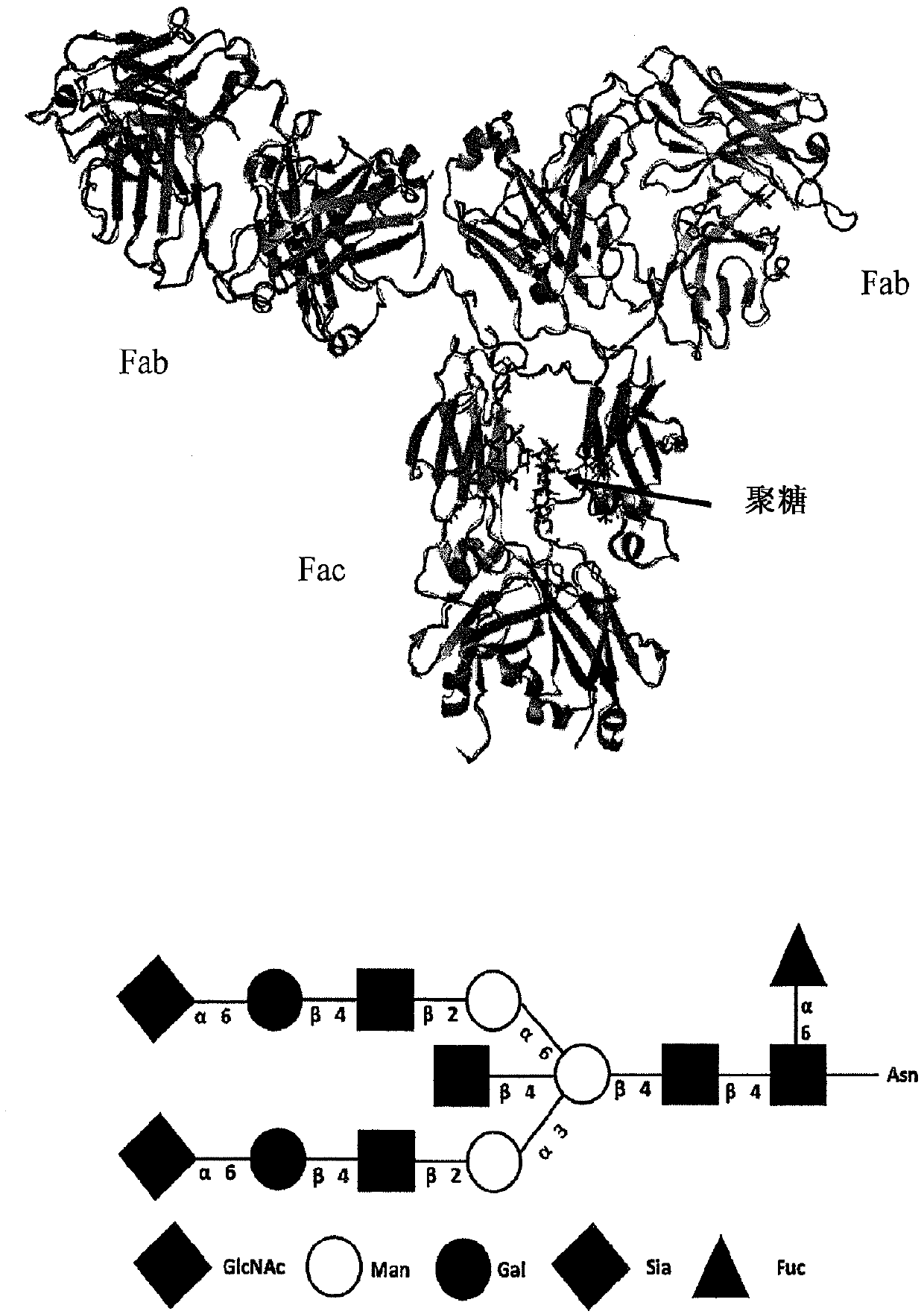
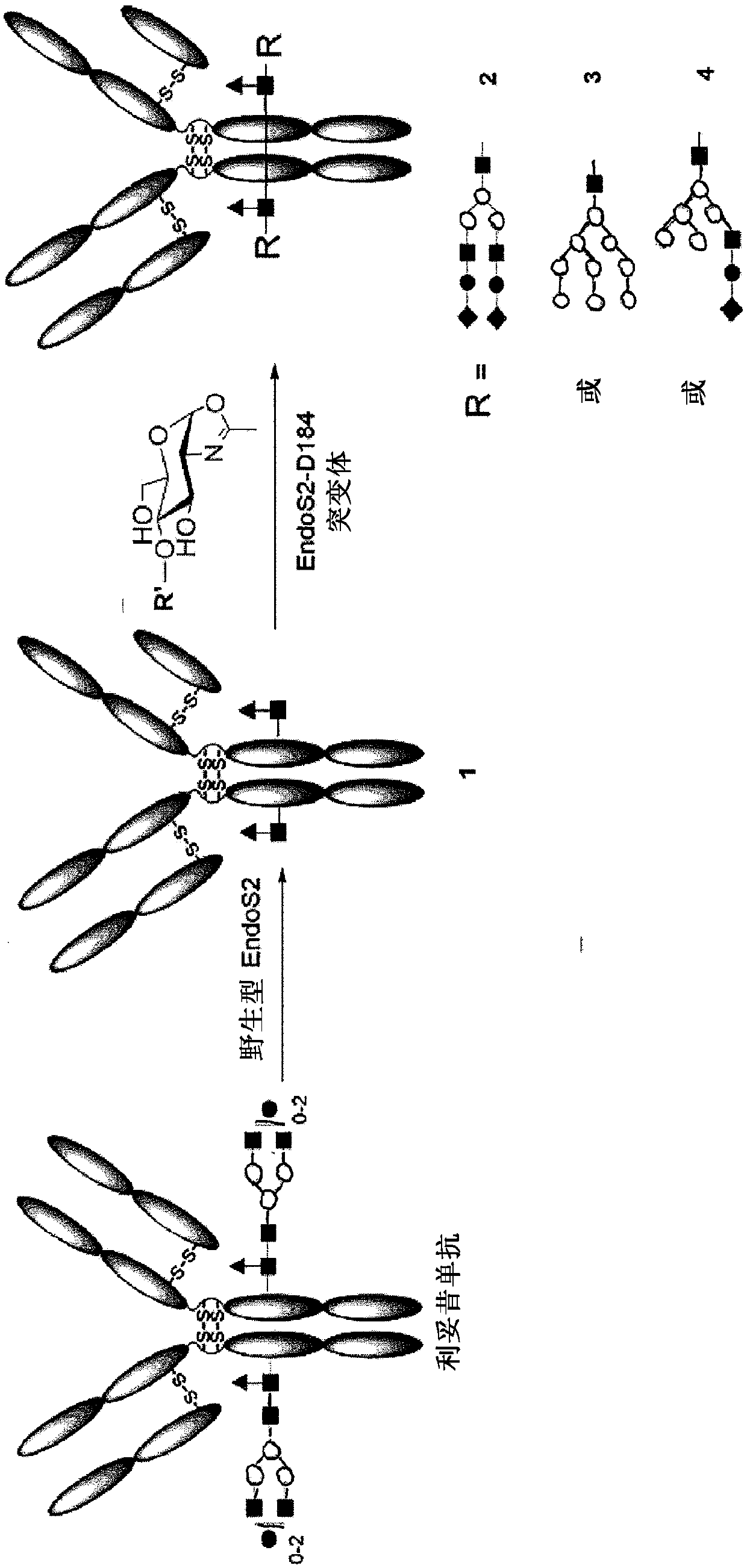
Endo-s2 mutants as glycosynthases, method of making and use for glycoengineering of glycoproteins
一种糖蛋白、突变体的技术,应用在糖蛋白合成领域,能够解决不清楚Endo-S2转糖基活性等问题,达到延长体内半衰期、提高靶向能力、小免疫原性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0115] Generation of Endo-S2 glycosynthase mutants and their use for glycosylation remodeling of the intact monoclonal antibody rituximab
[0116] Glycoside synthases have previously been shown to be responsible for promoting during hydrolysis oxazoline Site-directed mutagenesis of key asparagine (Asn) in the GH85 family or aspartic acid (Asp) residue in the GH18 family of ionic intermediates formed by several GH85 endoglycosidases (ENGases), described GH85 endoglycosidases include EndoA, EndoM, EndoD and GH18 endoglycosidase EndoS. [36,38] Endo-S2 is an endoglycosidase belonging to glycoside hydrolase family 18 (GH18) [33], which is in the same GH family as EndoS, EndoF1, EndoF2, and EndoF3, which were recently confirmed to have transglycosylation activity middle. Based on the hypothesis that EndoS2-catalyzed hydrolysis also involves the formation of oxazoline A substrate-assisted mechanism of ionic intermediates proceeds, as shown by other GH18 endoglycosidases such...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com