Drug eluting balloon
A balloon and drug technology, applied in medical science, balloon catheter, surgery, etc., can solve the problems of increasing cost and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0201] The test articles were coated with the same drug coating formulation and the same application method.
[0202] test article
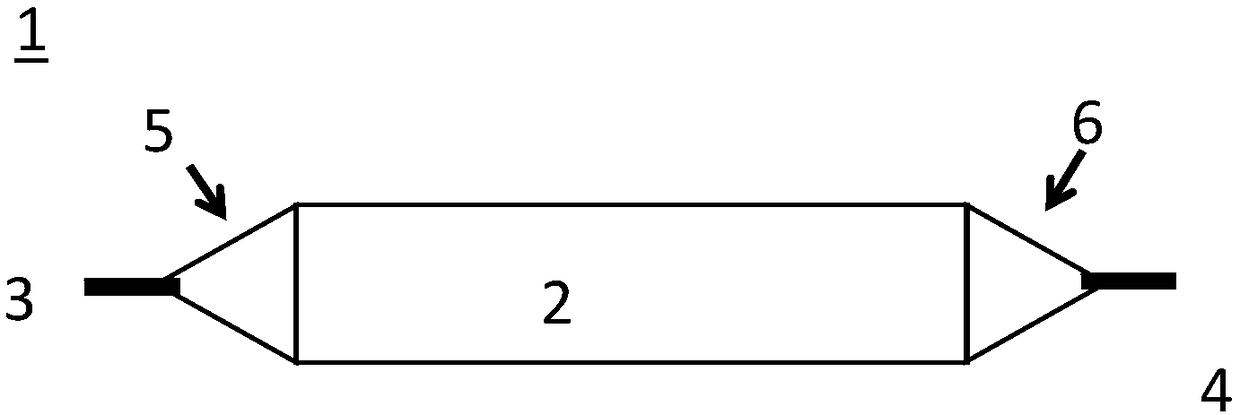
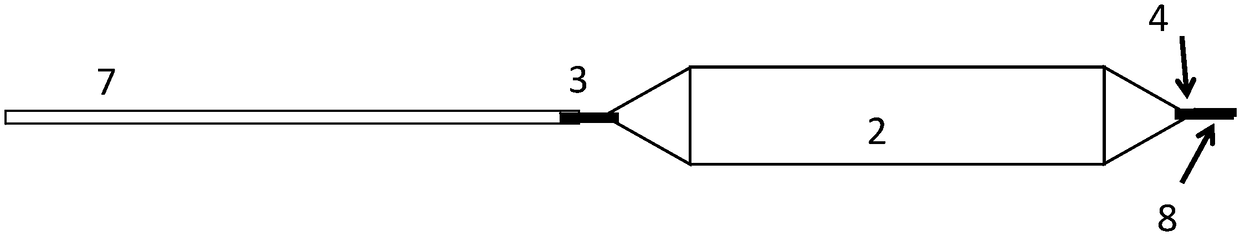
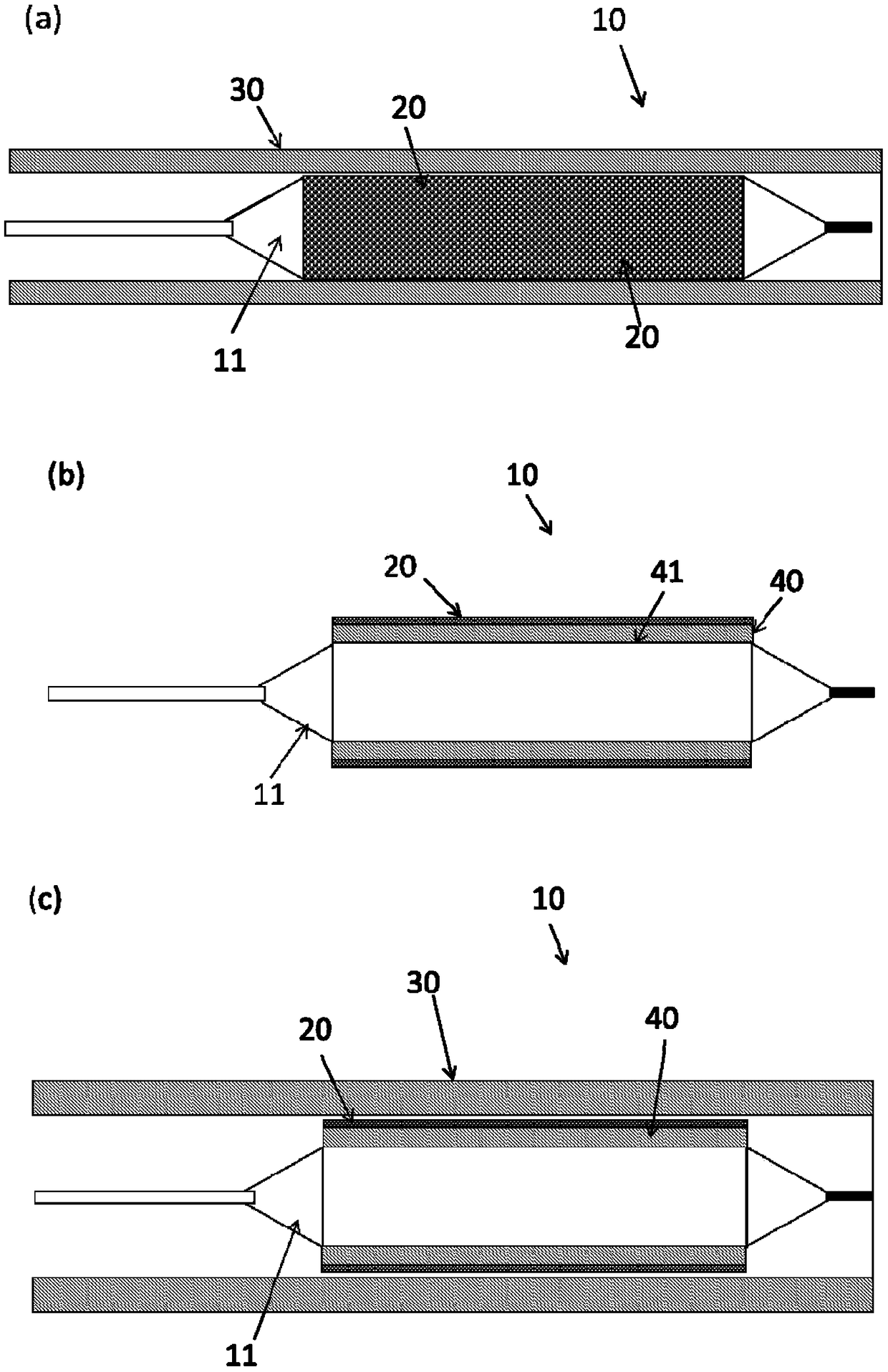
[0203] Device A is a drug delivery device prepared similarly to Figure 3(a).
[0204] Device B is a drug delivery device prepared similarly to Figure 10(c).
[0205] Device C is a drug delivery device prepared similarly to Figure 10(a).
[0206] Device D is a typical balloon catheter.
[0207] method
[0208] In vitro test method adapted from Seidlitz et al. (2013 In Vitro Determination of Drug Transfer from Drug-Coated Balloons PLoS ONE8(12):e83992(doi:10.1371 / journal.pone.0083992).)
[0209] The following adaptations were made:
[0210] ·The wall of the model container is made of silicon tube.
[0211] • Imaging of the model vessel wall was not required, so the balloon was not treated with fluorescent substances.
[0212] ·Use ACN to extract the drug content and analyze it with a UV spectrophotometer at 227nm.
[0213] • The balloon is ...
specific Embodiment 2
[0217] The test articles were coated with the same drug coating formulation and the same application method.
[0218] test article
[0219] Device C is a drug delivery device prepared similarly to Figure 10(a).
[0220] Device D is a typical balloon catheter.
[0221] method
[0222] In vitro test method adapted from Seidlitz et al. (2013 In Vitro Determination of Drug Transfer from Drug-Coated Balloons PLoS ONE8(12):e83992(doi:10.1371 / journal.pone.0083992).)
[0223] The following adaptations were made:
[0224] ·The wall of the model container is made of silicon tube.
[0225] ·Shorten the transportation route.
[0226] • Imaging of the model vessel wall was not performed, so the balloon was not treated with fluorescent substances.
[0227] • Extract drug content in ACN and analyze using UV spectrometer at 227nm.
[0228] • The balloon is under a pressure of 12ATM.
[0229] result
[0230] Such as Figure 13 As shown, Device C with an elastic membrane, has signific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap