Sequence arrangements and sequences for neoepitope presentation
A new epitope and sequence technology, applied in the field of immunotherapy compositions, can solve the problems of unpredictable treatment success and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
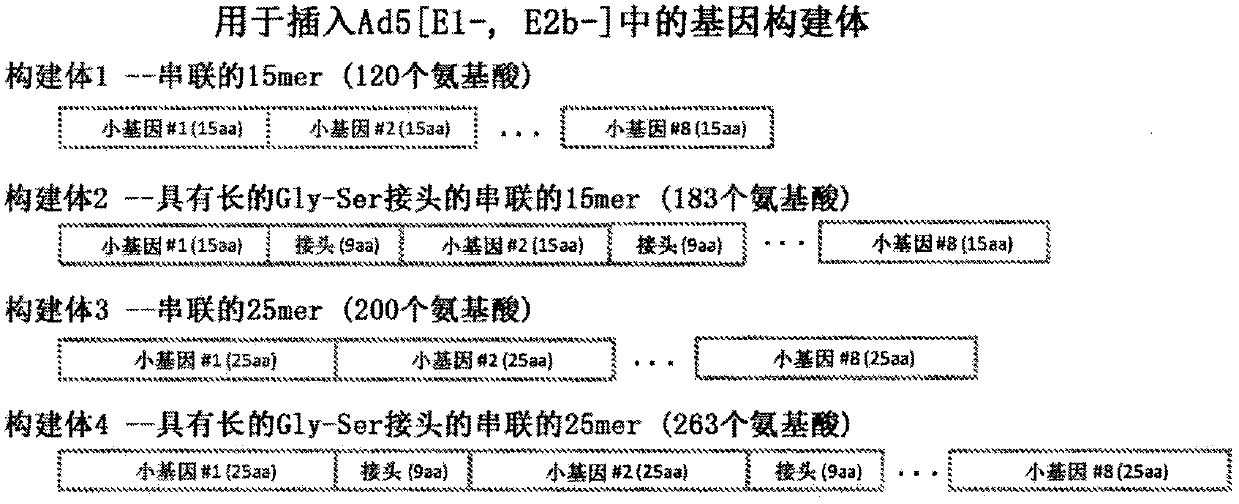
[0107] Exemplary sequence alignment
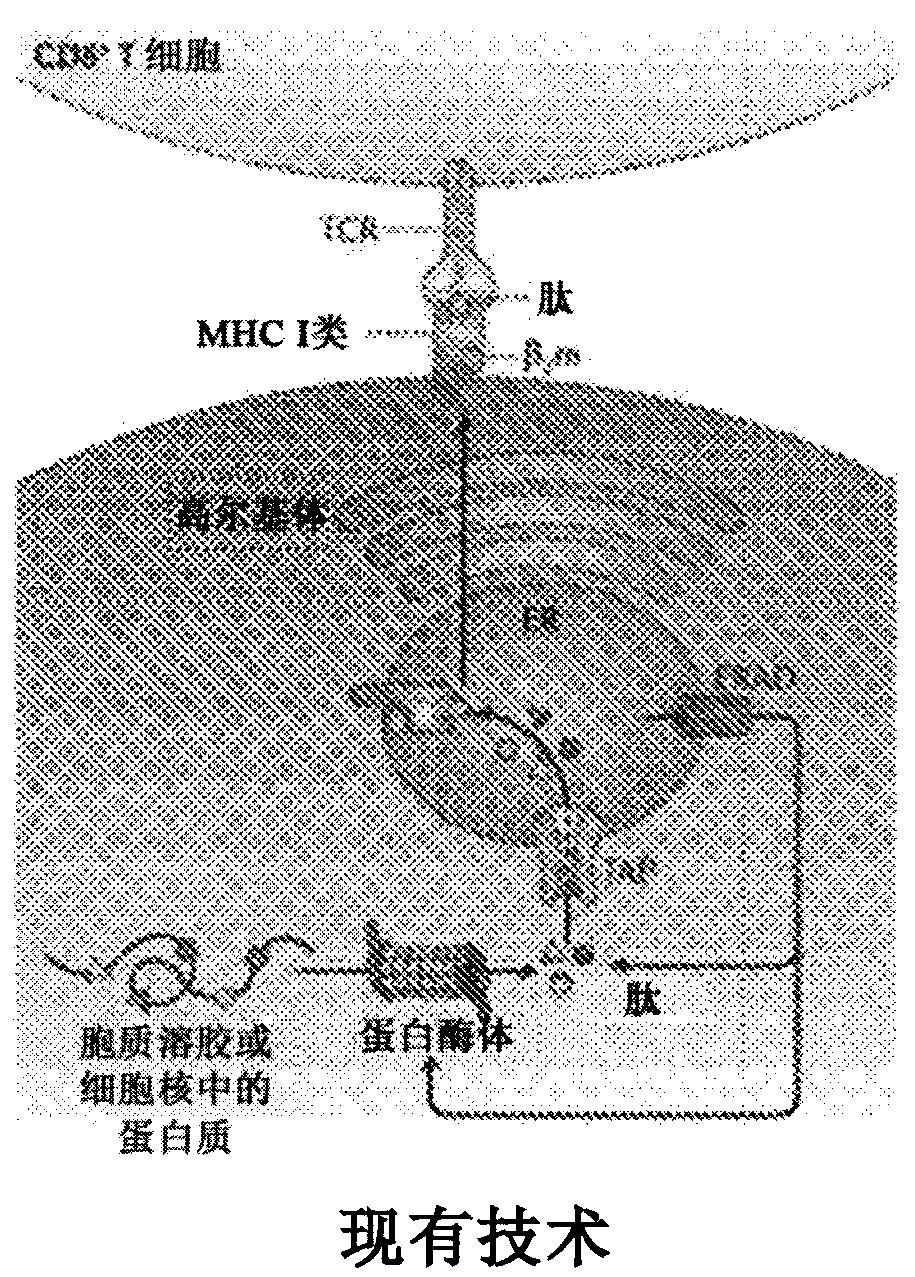
[0108] Neo-epitope sequences were determined via in silico by position-directed simultaneous alignment of tumor and normal samples, eg as disclosed in US2012 / 0059670 and US2012 / 0066001 using BAM files and BAM server. Specifically, DNA analysis of tumors was from the B16-F10 mouse melanoma line and matching normal tissue was blood DNA from C57bl / 6 parental mice. Expression results were screened by RNA sequencing of this tumor cell line. The found expressed neo-epitopes were further analyzed for binding affinity to murine MHC-I (here: Kb) and MHC-II (here: I-Ab). Following the further steps of dbSNP screening using the positional alignment of all neo-epitopes, followed by a weighting matrix and neural network prediction processing to generate scores representing the likelihood and / or strength of the presence of hydrophobic sequences or signal peptides, further analysis of the selected Binders (with an affinity equal to or less than 200 nM)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com