Clinical test document management system and management method
A document management system and clinical trial technology, which is applied in the field of clinical trial document management system and management, can solve the problems of clinical trial data line inspection and questioning difficulties, achieve effective positioning and query, and improve the efficiency of document management
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
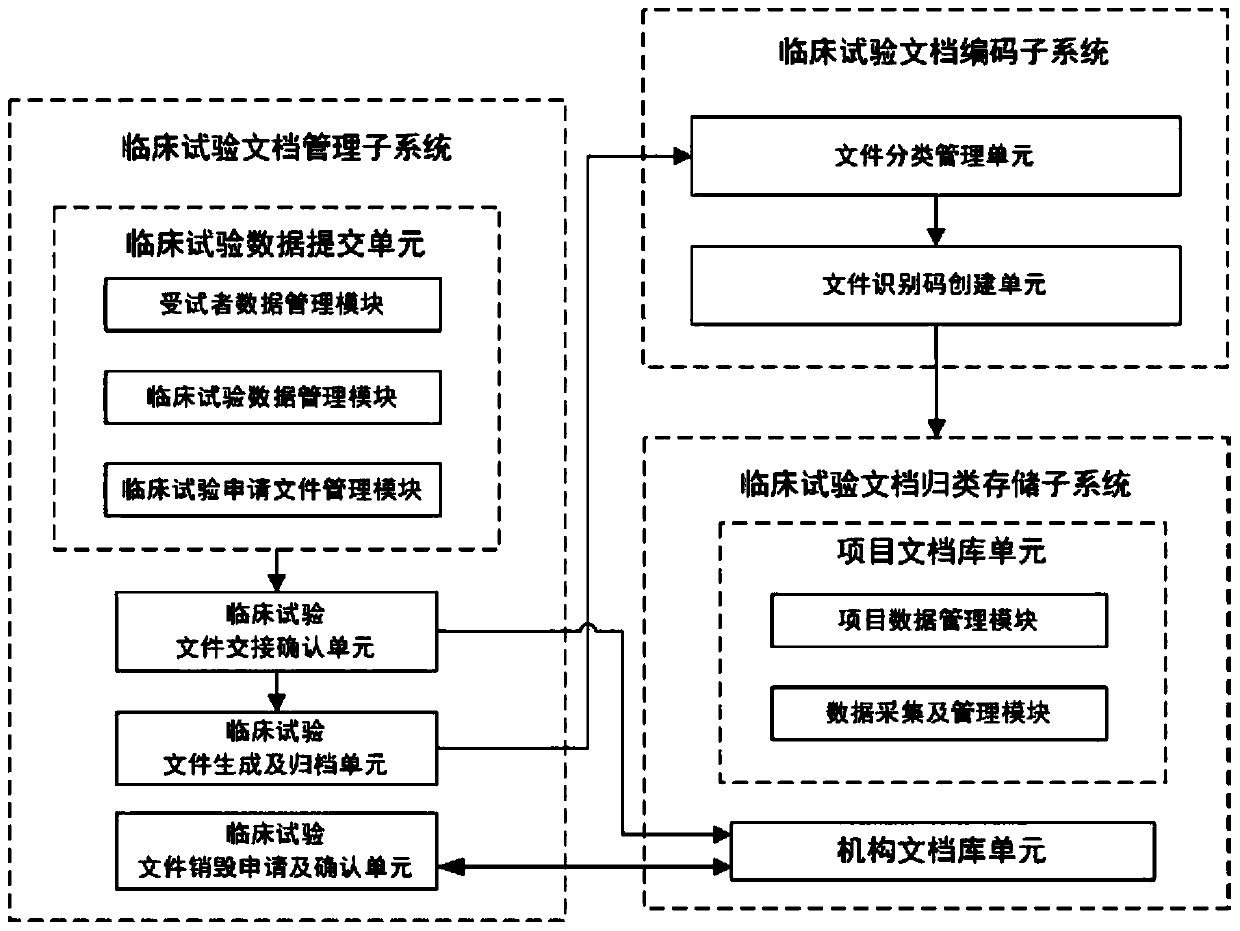
[0033] see figure 1 , a schematic structural diagram of a clinical trial document management system provided by an embodiment of the present invention. The clinical trial document management system provided by the embodiment of the present invention includes: a clinical trial document coding subsystem, a clinical trial document classification storage subsystem, and a clinical trial document management subsystem; wherein, the clinical trial document management subsystem is used to collect clinical trial documents Relevant documents and data, generate clinical trial documents, and its output terminal is connected with the input terminal of the clinical trial document coding subsystem; the clinical trial document coding subsystem adopts the institutional / professional code mechanism to classify the documents and compile the corresponding Unique document identification code; the clinical trial document classification storage subsystem classifies and stores the coded documents.
[...
Embodiment 2
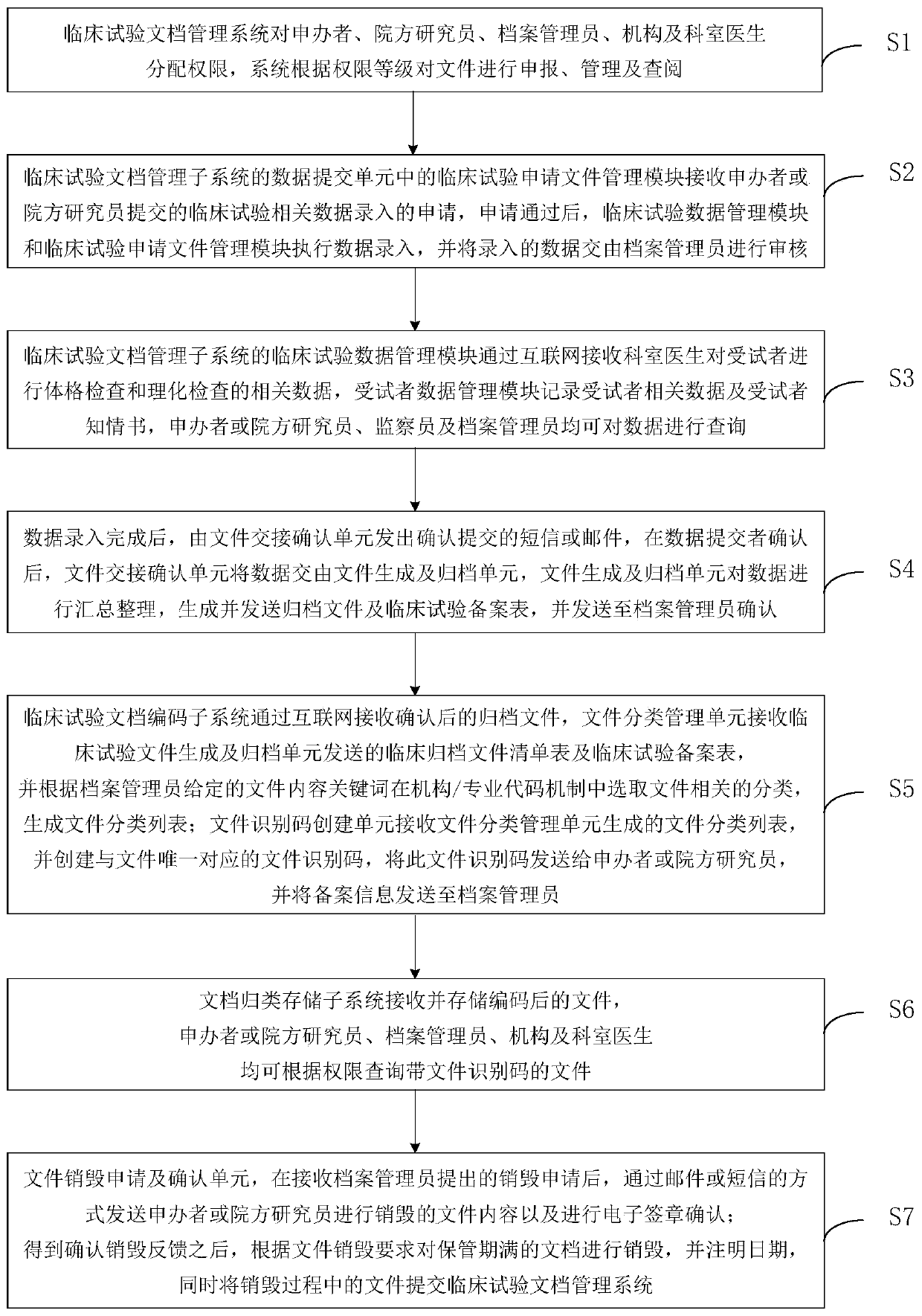
[0049] see figure 2 , a flowchart of a clinical trial document management method provided by an embodiment of the present invention. The clinical trial document management method that the embodiment of the present invention provides, comprises the following steps:
[0050] (1) The clinical trial document management system assigns authority to sponsors, hospital researchers, archivists, institutions and department doctors, and the system declares, manages and consults documents according to authority levels;
[0051] (2) The clinical trial application document management module in the data submission unit of the clinical trial document management subsystem receives the application for clinical trial data input submitted by the sponsor or the hospital researcher. After the application is approved, the clinical trial data management module and clinical trial The application file management module performs data entry, and submits the entered data to the file administrator for re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com