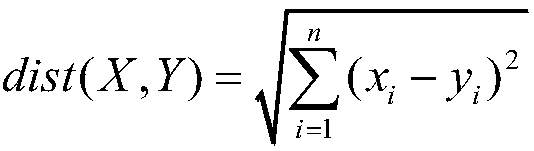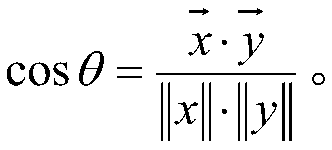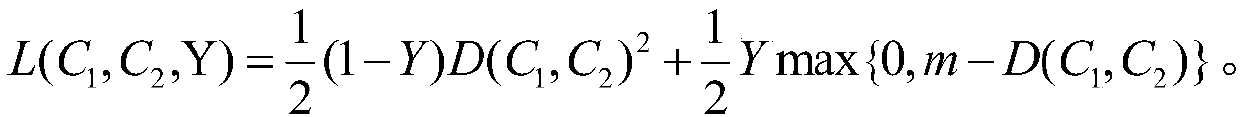Chemical reaction validity predicting method
A technique for chemical reactions and prediction methods, applied at the intersection of computer science and chemical organic synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] In order to make the technical means, creative features, goals and effects achieved by the present invention easy to understand, the above technical solutions are further described below.
[0019] A method for predicting the legitimacy of chemical reactions considers each chemical element as a word and builds an element table containing M words. In order to enable the model to automatically segment elements, manually labeled segmentation data can be used to pre-train the segmentation model to segment elements. The splitting method includes splitting by periodic table elements and splitting by compounds.
[0020] After the elements are segmented, use word embedding (Word Embedding) method to map each element to a vector space of specific dimension N, and at this time obtain an M×N mapping space (Embedding). Assuming that a response contains K elements, the response is expressed as a vector of (K, M, N) dimensions.
[0021] The existing chemical reactions are divided in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com