Compositions and methods for the treatment of myotonic dystrophy
A genome and repetitive sequence technology, applied in the field of compositions for the treatment of myotonic dystrophy, can solve the problems of unsuitable removal of trinucleotide repeat sequence expansion regions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
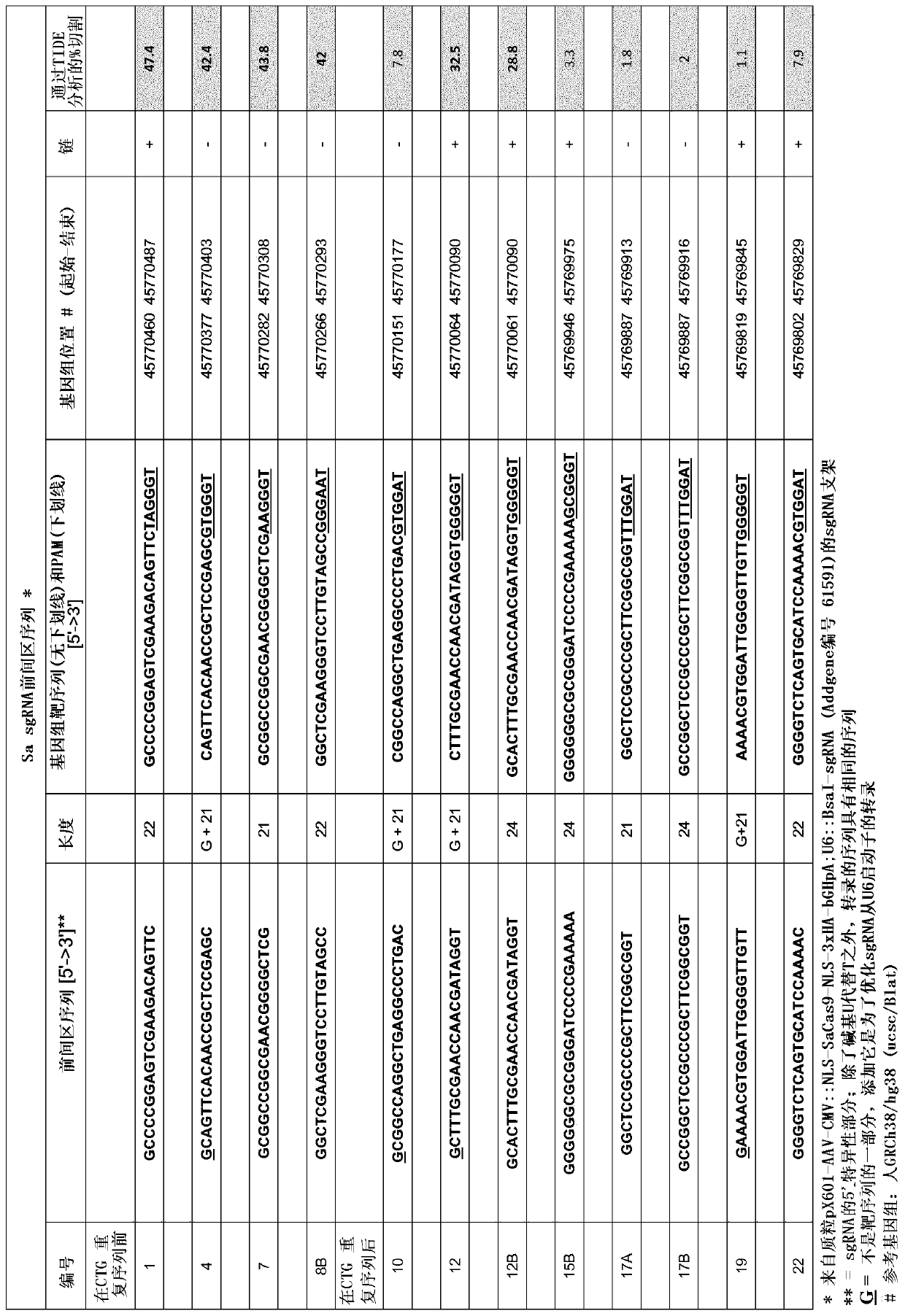
[0138] A table matching the SEQ ID NOs to the sgRNA numbers used in the experimental section and figures below is provided below.
[0139] sgRNA ID 1 4 7 8B 12 12B SEQ ID NO 1 20 3 4 21 6
[0140] Materials and methods
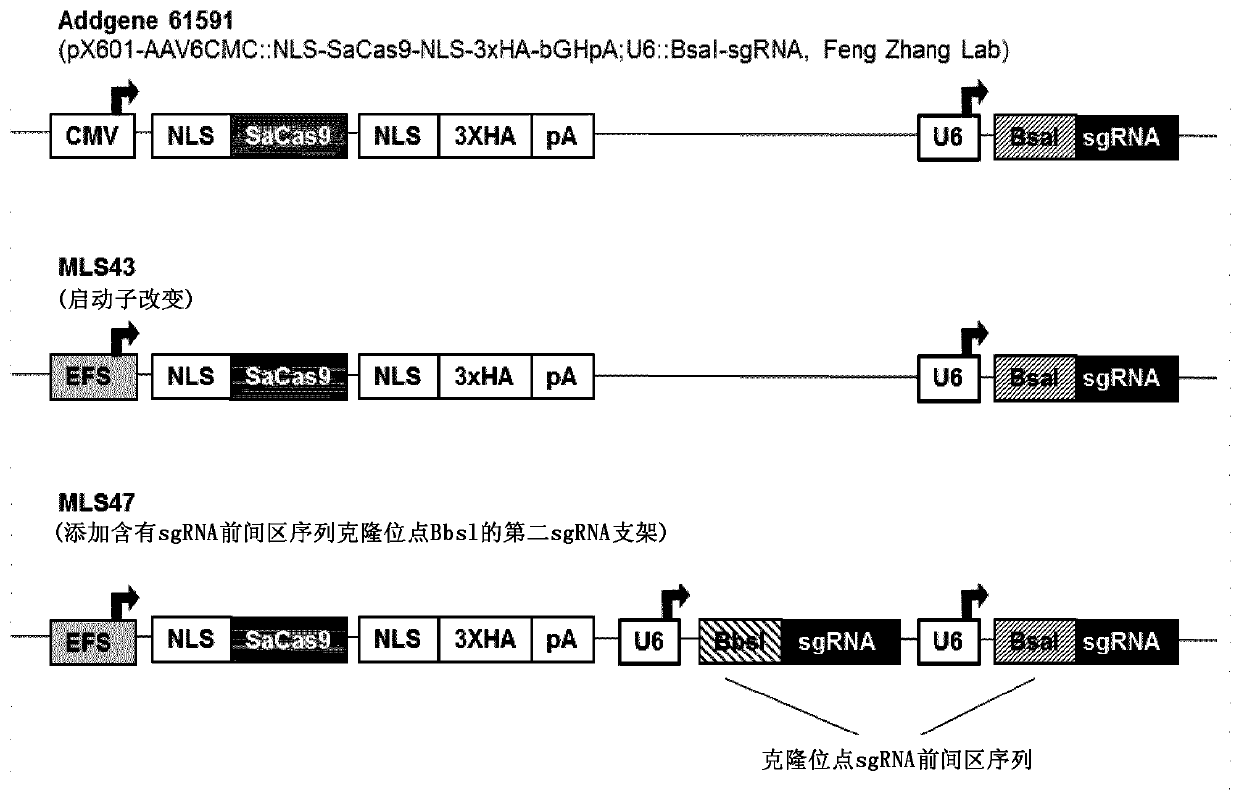
[0141] Plasmid construction
[0142] The plasmid encoding S. aureus Cas9 was derived from the plasmid pX601-AAV-CMV::NLS-SaCas9-NLS-3xHA-bGHpA;U6::BsaI-sgRNA(MLS42) [Ran et al., 2015]. The EFS promoter was PCR amplified with primers F-XhoI-MreI-EFS (MLS63) and R-XmaI-NruI-EFS (MLS64) and cloned into the promoterless pX601-AAV-::NLS-SaCas9- NLS-3xHA-bGHpA;U6::BsaI-sgRNA XhoI / AgeI sites to obtain pAAV-EFS::NLS-SaCas9-NLS-3xHA-bGHpA;U6::BsaI-sgRNA(MLS43).
[0143] The second sgRNA cassette (U6::BbsI-sgRNA) was cloned in tandem into the Acc65I site upstream of the first sgRNA cassette in plasmid MLS43 to obtain construct pAAV-EFS::NLS-SaCas9-NLS-3xHA-bGHpA; U6::BbsI-sgRNA; U6::BsaI-sgRNA (MLS47). Insert U6::BbsI-sgRNA was synt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com