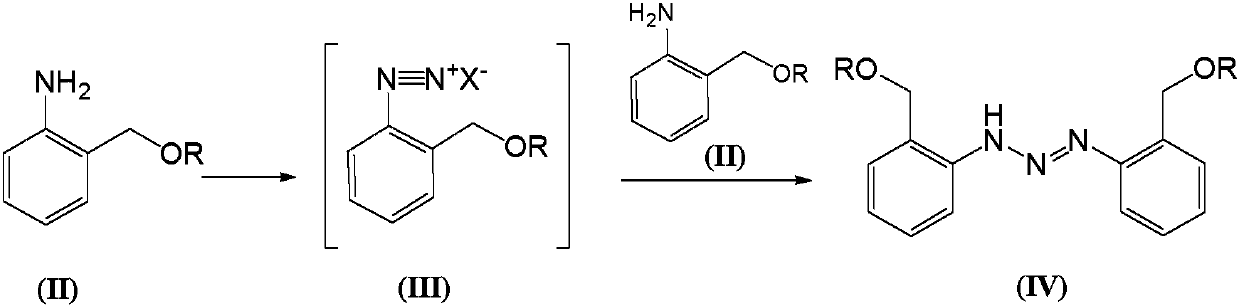
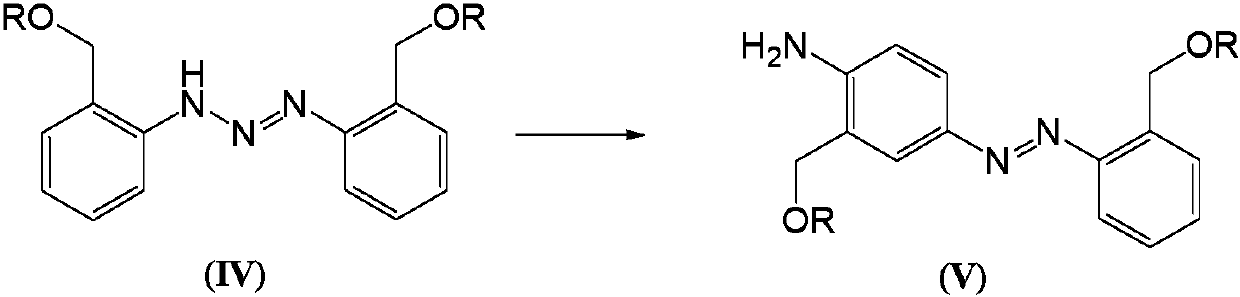
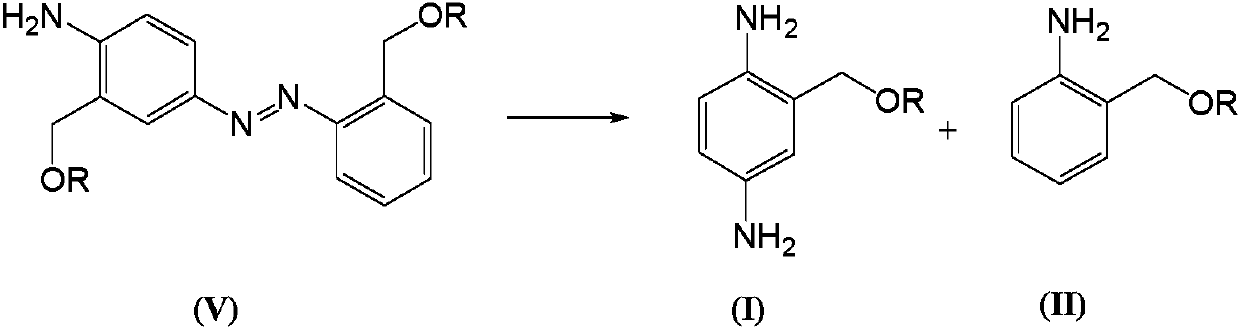
Telescoping synthesis of 2-methoxymethyl-p-phenylenediamine
A technology of phenylenediamine and aniline, which is applied in the field of preparing 2-substituted-1, and can solve the problems of unsatisfactory synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0083] The following are non-limiting examples of compositions of the invention. These examples are given for the purpose of illustration only and are not to be construed as limiting the invention, since many variations thereof are possible without departing from the spirit and scope of the invention, which will be recognized by ordinary skill in the art recognized by personnel. All concentrations are listed in percent by weight unless specifically stated otherwise.
[0084] 1.1 Placing 2-nitrobenzyl alcohol (VI) to start to prepare 2-methoxymethyl-nitrobenzene (VII)
[0085] 2-Nitrobenzyl alcohol (100 g, 0.653 moles) was partitioned between 50% NaOH (136 g, 1.7 moles) and petroleum ether (500 mL). Via addition funnel (moderate rate), benzyltriethylammonium chloride (2 g) was added followed by dimethyl sulfate (106.4 g, 0.84 mol). The mixture was stirred (mechanically) until complete. Dimethyl sulfate (10 mL) was added and the reaction was stirred for 1 hour. Excess dim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com