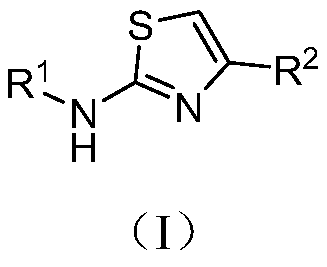
Aminothiazole compound as well as preparation method and application thereof in resisting enterovirus 71
A technology of aminothiazole and enterovirus, which is applied in the field of medicine to achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] [Example 1] Preparation of aromatic heterocyclic thiourea derivatives with substituent R
[0030] The aromatic heterocyclic thiourea derivatives with R are synthesized by the reaction shown in the following formula i.
[0031]
[0032] Taking the preparation of 1-(2,6-dibromophenyl)thiourea 2m as an example, the steps are as follows: Dissolve ammonium thiocyanate (273.0mg, 3.6mmol) in 15mL of acetone, then add benzoyl chloride (460.2mg , 3.38mmol), reflux for 15min. Cool to room temperature, add 2,6-dibromoaniline 1m (600mg, 2.4mmol), and reflux for 30min. After cooling to room temperature, pour into ice water and stir for 30 min. Let stand for two minutes, filter with a suction filter, wash the precipitate with water, dissolve the solid in 15mL of 10% NaOH solution after drying, heat and stir at 80°C for 30min, cool to room temperature, slowly add 6N hydrochloric acid dropwise until the pH of the solution is 1- 2. Stir for 30 minutes, then adjust the pH to 10 wit...
Embodiment 2
[0034] [Example 2] with R 2 Preparation of α-Chloroacetone Derivatives
[0035] Synthesized by the reactions shown in the following formulas ii and iii to obtain with R 2 α-chloroacetone derivatives.
[0036]
[0037] Taking the preparation of 2-chloro-1-(imidazo[1,2-a]pyrimidin-3-yl)ethan-1-one 5a as an example, the steps are as follows: Weigh 2-aminopyrimidine 3a (1g, 10.5mmol) Dissolve in 20mL of toluene, add N,N-dimethyl dimethyl acetal (1.38g, 11.6mmol), and reflux for 6h. After the completion of the reaction was monitored by TLC spotting, the reaction was spin-dried to obtain the crude product N,N-dimethyl-N'-(pyrimidin-2-yl)formamidine 4a. Dissolve it in 15 mL of dichloromethane, add 1,3-dichloroacetone (2 g, 15.8 mmol), and reflux overnight. After the completion of the reaction was monitored by TLC, it was concentrated and purified through a silica gel column with a mobile phase ratio of petroleum ether and ethyl acetate (V / V=1 / 3). Finally, 700 mg of 2-chloro-1...
Embodiment 3
[0040] [Example 3] Preparation of aminothiazole compounds of the present invention
[0041] Aminothiazole compounds are synthesized through reactions shown in the following formulas iv and v.
[0042] Take 1.0 equivalent of the aromatic heterocyclic thiourea derivative prepared in Example 1 and place it in a 25 mL single-necked bottle, add 8 mL of absolute ethanol, then add 1.0 equivalent of the compound prepared or purchased in Example 2, and reflux overnight. The reaction was monitored by TLC, and after confirming that the reaction was complete, aminothiazole compounds were obtained by separation and purification by silica gel column chromatography. The reaction formula is as follows:
[0043]
[0044] R 1 for
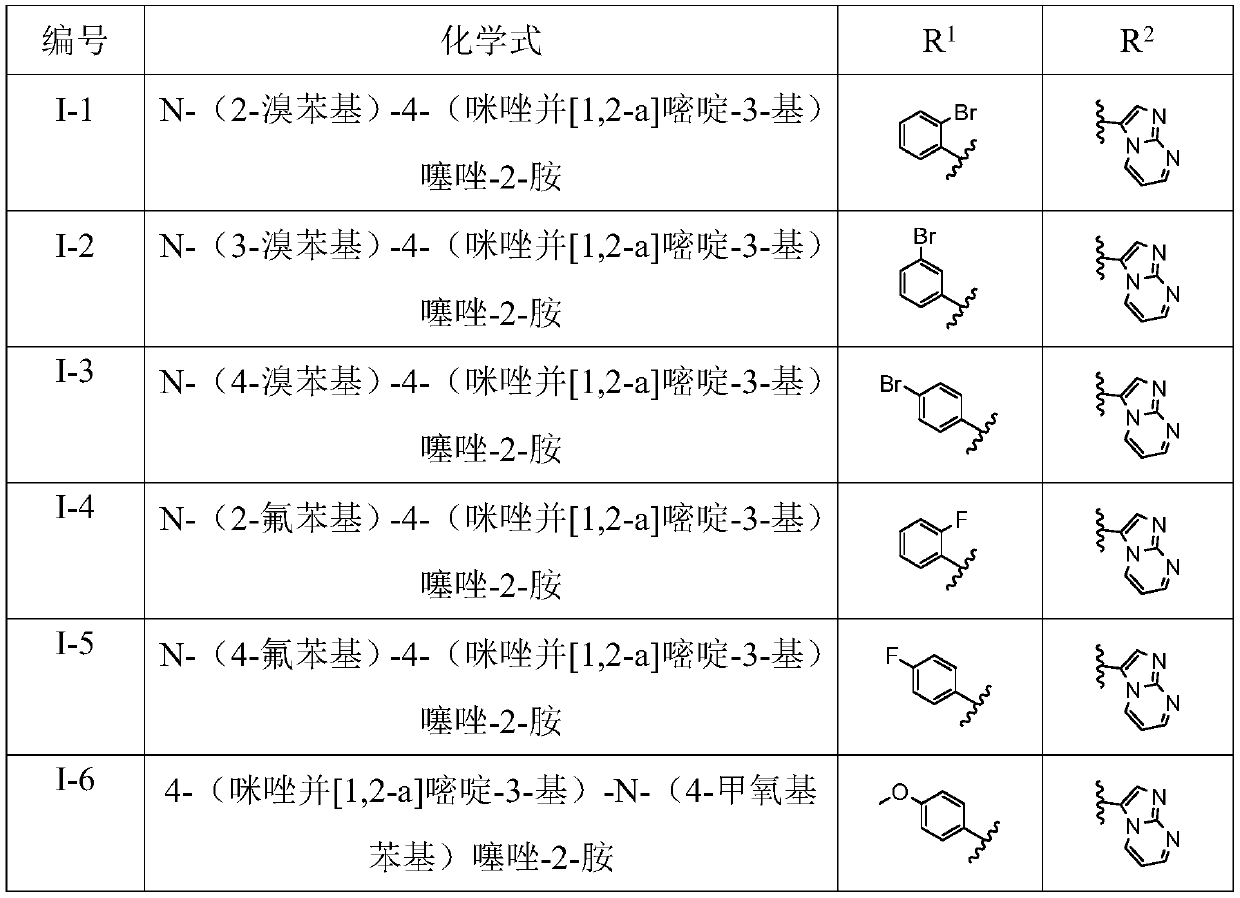
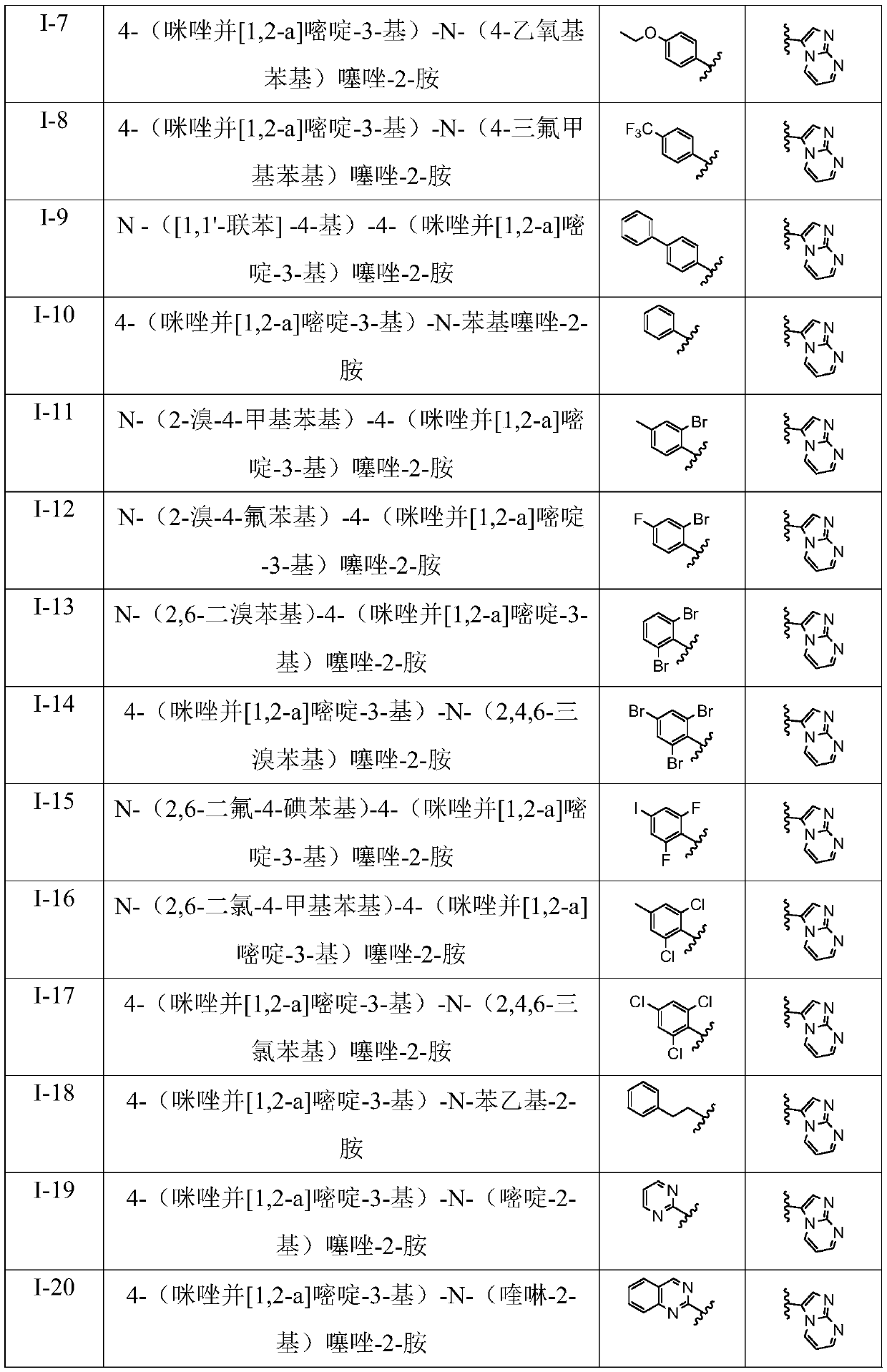
[0045] R 2 for 6a-6x above correspond to I-1 to I-24 in Table 1.
[0046](1) Preparation of N-(2-bromophenyl)-4-(imidazo[1,2-a]pyrimidin-3-yl)thiazol-2-amine (I-1) Compound 2a N-(2 The preparation of -bromophenyl)-4-(imidazo[1,2-a]pyrimidin-3-yl)thiazo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com