Novel uses of piperidinyl-indole derivatives
A technology of indole and methyl, used in the field of treating patients with C3G and IgAN, can solve the problems of no description of application, no description of piperidinyl-indole derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
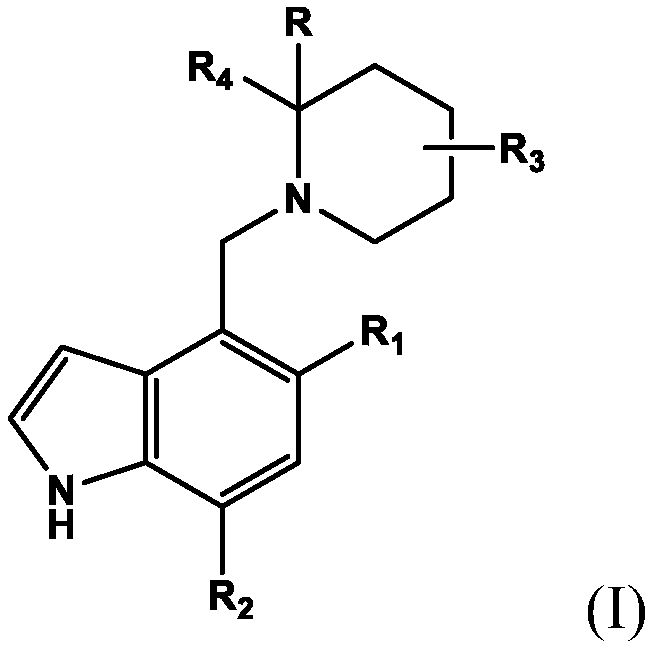
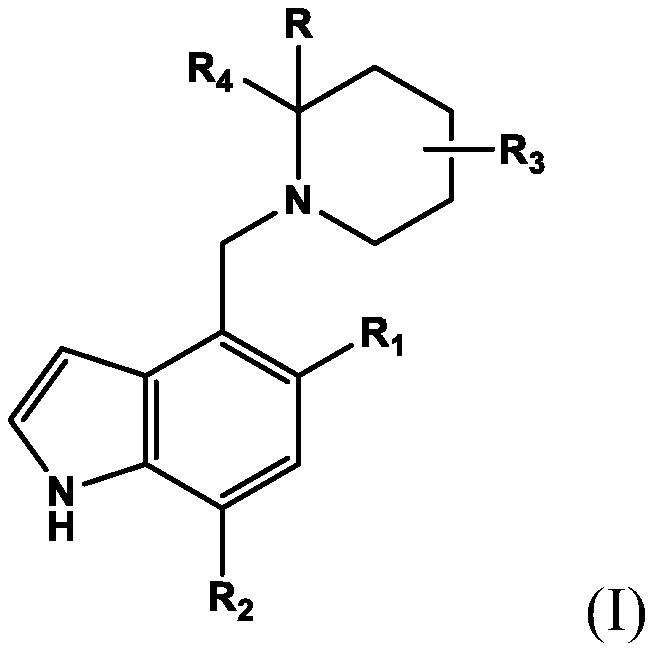
[0007] In the first embodiment, the present invention provides a compound of formula (I) or a pharmaceutically acceptable salt thereof for use in the treatment of complement-driven renal disease C3G (C3 glomerulopathy), IgAN (immunoglobulin) A nephropathy) and other nephropathy with signs of glomerular C3 deposition (such as MN (membranous nephropathy) and HUS (E. coli-induced hemolytic uremic syndrome)). The compound of formula (I) or a pharmaceutically acceptable salt thereof is represented by the following structure:
[0008]
[0009] among them
[0010] R is hydrogen, C 1 -C 4 Alkyl, or C 1 -C 6 Alkoxy;
[0011] R 1 Is C 1 -C 6 Alkoxy;
[0012] R 2 Is C 1 -C 6 alkyl;
[0013] R 3 Is C 1 -C 6 Alkoxy; C 1 -C 6 Alkyl; or hydroxyl;
[0014] R 4 Is optionally -C(O)R 8 Substituted phenyl, and
[0015] R 8 Is hydroxyl, C 1 -C 4 Alkoxy or amino.
[0016] In a second embodiment, the present invention provides a compound for use according to the first embodiment or a pharmaceutically acceptabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com