Anti igf, anti pd-1 Anti-cancer combination therapy
A composition and antibody technology, applied in the direction of drug combination, antibody medical components, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
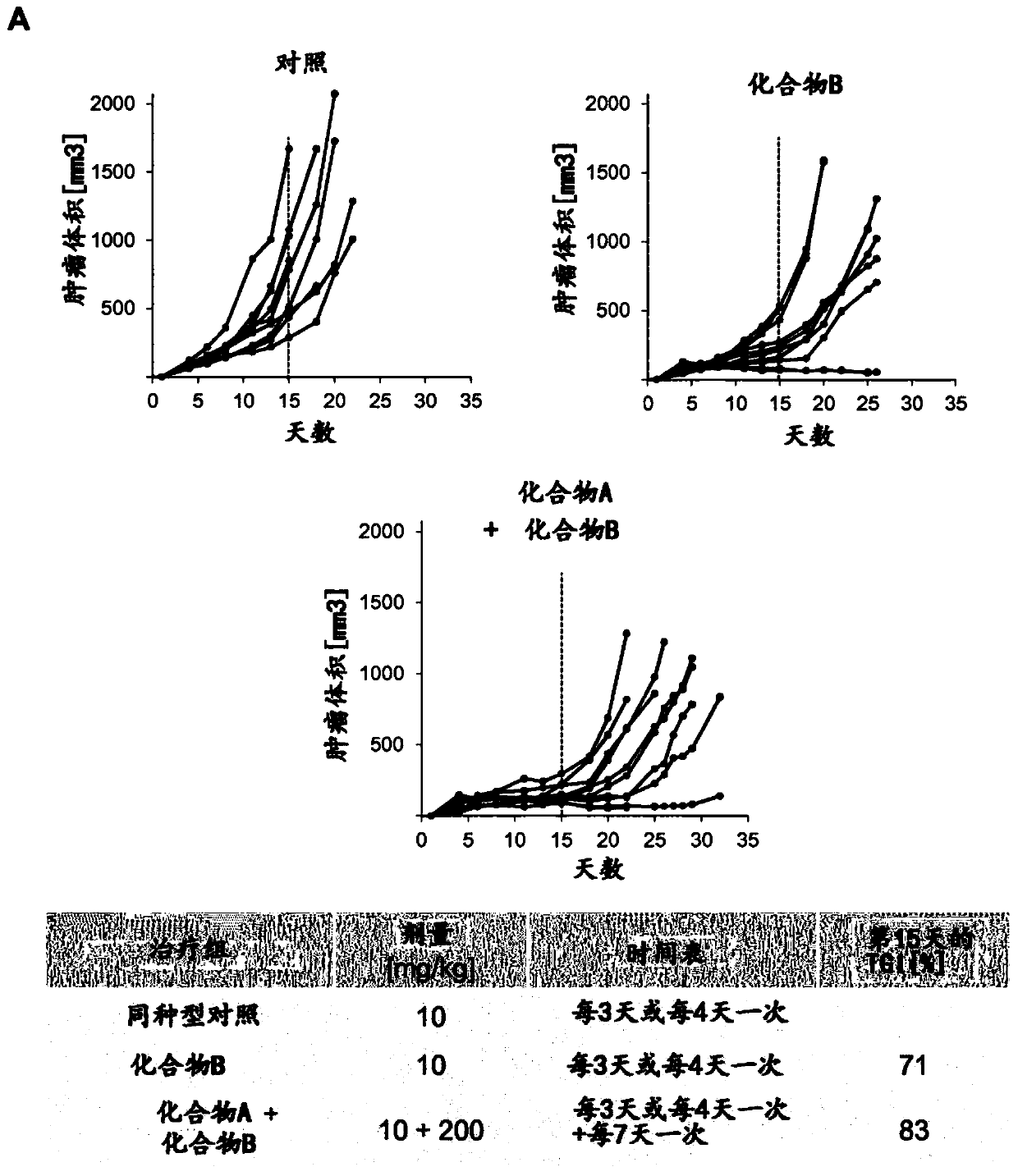
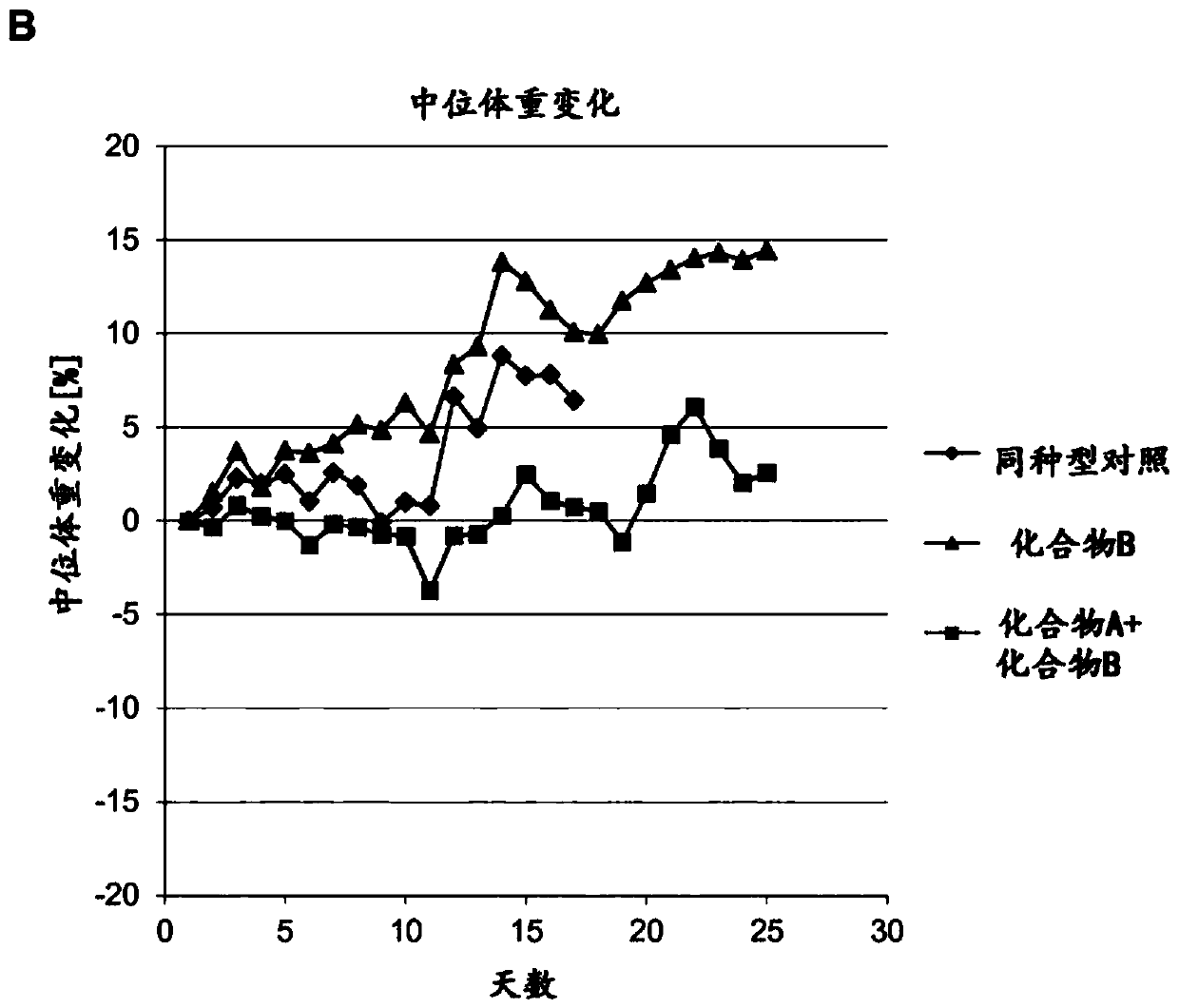
[0200] In Vivo Antitumor Efficacy of the Combination of Compound A and Compound B in a Colon Cancer Model
[0201] Materials and methods
[0202] The antitumor efficacy of the combination of Compound A and Compound B was investigated in a syngeneic mouse tumor model derived from the murine colon carcinoma cell line MC-38. Tumor cells were implanted subcutaneously into immunocompetent syngeneic C57Bl / 6 6- to 8-week-old female mice (in 30 μl Matrigel, 1 x 10 6 tumor cells) and established tumors three days before starting treatment. Compound B (PD-1 mouse antibody, 10 mg / Kg, once every three or four days), compound B (PD-1 mouse antibody, 10 mg / Kg, every three or four days) were administered intraperitoneally (ip) to mice. once a day) and compound A (BI-IGF, 200mg / Kg, once every seven days) or IgG isotype control antibody (10mg / Kg, once every three or four days) for 15-32 days. Tumor volumes were measured three times a week using calipers. Calculate the volume of each tumor ...
Embodiment 2
[0210] In Vivo Antitumor Efficacy of the Combination of Compound A and Compound B in a Breast Cancer Model
[0211] Materials and methods
[0212] The antitumor efficacy of the combination of Compound A and Compound B was investigated in a syngeneic mouse tumor model derived from the breast cancer cell line EMT-6. Tumor cells were implanted subcutaneously into immunocompetent syngeneic BALB / c 6- to 8-week-old female mice (in 30 μl Matrigel, 1 x 10 6 tumor cells) and establish tumors 6 to 10 days before starting treatment. Compound B (PD-1 mouse antibody, 10 mg / Kg, once every three or four days), compound B (PD-1 mouse antibody, 10 mg / Kg, every three or four days) were administered intraperitoneally (ip) to mice. once a day) and compound A (BI-IGF, 200mg / Kg, once every seven days) or IgG isotype control antibody (10mg / Kg, once every three or four days) for 10-35 days. Tumor volumes were measured three times a week using calipers. Calculate the volume of each tumor [mm] acco...
Embodiment 3
[0215] Effects of Compound A and Compound B Combination on Intratumoral Regulatory T Cells (Tregs) in a Colon Cancer Model
[0216] Materials and methods
[0217] In a syngeneic mouse tumor model derived from the colon cancer cell line MC-38, the reduction of intratumoral Treg following combined treatment with compound A and compound B was investigated. Tumor cells were implanted subcutaneously into immunocompetent syngeneic C57Bl / 6 6- to 8-week-old female mice (in 30 μl Matrigel, 1 x 10 6 tumor cells) and establish tumors 3 to 6 days before starting treatment. Intraperitoneal (ip) administration of compound A (BI-IGF, 200mg / Kg, once every seven days), compound B (mouse antibody to PD-1, 10mg / Kg, once every three or four days), compound Combination of B (PD-1, 10mg / Kg, once every three or four days) and compound A (BI-IGF, 200mg / Kg, once every seven days), or IgG isotype control antibody (10mg / Kg, every once every three or four days) for 10-35 days. Tumor volumes were meas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com