Methods and compositions for treating inflammatory skin disease with recombinant microorganisms
A technology for recombining microorganisms and compositions, applied in skin diseases, drug combinations, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Example 1: Development of a nucleic acid construct that encodes a protein that can be exported from S. epidermidis cells and then imported into human keratinocytes
[0146] In one embodiment, the present invention describes the production of recombinant S. epidermidis strains capable of secreting heterologous proteins, thus overcoming the intractability of genetic modification of S. epidermidis. Previous functional gene analyzes of the common skin colonizers S. aureus and S. epidermidis have been limited by the presence of type I and type IV restriction systems in nearly all strains of these bacteria. These restriction systems recognize methylated cytosine bases in DNA from standard clonal expansion systems such as DH10B E. coli. However, several constructs have been created in S. epidermidis strain ATCC12228 using the methylation-deficient E. coli strain DC10B 5, the strain is a commensal non-pathogenic isolate that lacks the ica operon. Thus, the present invention...
Embodiment 2
[0151] Example 2: Determination of Persistence and Localization of Topically Applied Staphylococcus Epidermidis Using an In Vitro Model System
[0152] Materials and methods
[0153] Build reporter bacteria
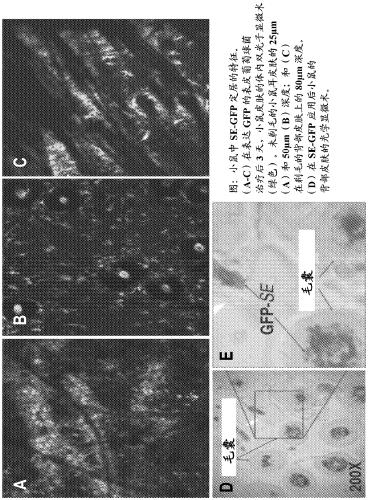
[0154] To facilitate tracking of topically applied bacteria, a S. epidermidis (SE) strain expressing sGFP was used. remove SecA and RMR peptide so that sGFP protein does not shuttle into the secretion system and free sGFP does not penetrate the stratum corneum. This construct is called SE-sGFP .
[0155] Quantify and compare the growth characteristics of transformed bacteria in liquid media
[0156] A basic understanding of the ability of transformed (recombinant) S. epidermidis to compete with wild-type S. epidermidis is required. To understand the growth characteristics of transformed bacteria as well as the growth kinetics of recombinant protein producing bacteria, colony forming units (CFU) in liquid media were quantified using standard techniques. Staphylo...
Embodiment 3
[0164] Example 3: Characterization of delivery of bacterially secreted sGFP to skin using an in vitro model system
[0165] Characterization of sGFP production in SE
[0166] Characterization of delivery of bulk purified sGFP and sGFP+RMR to RHE
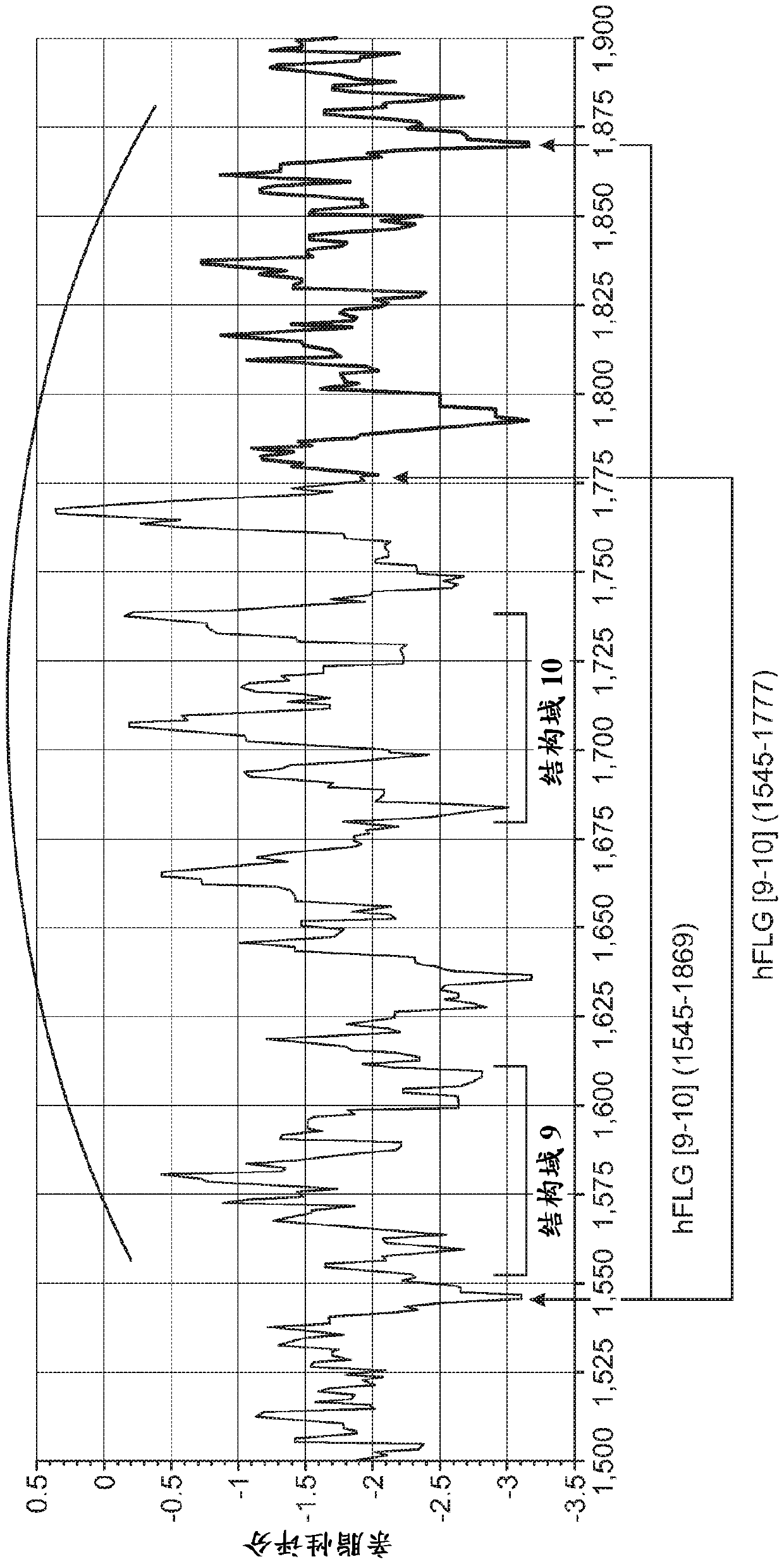
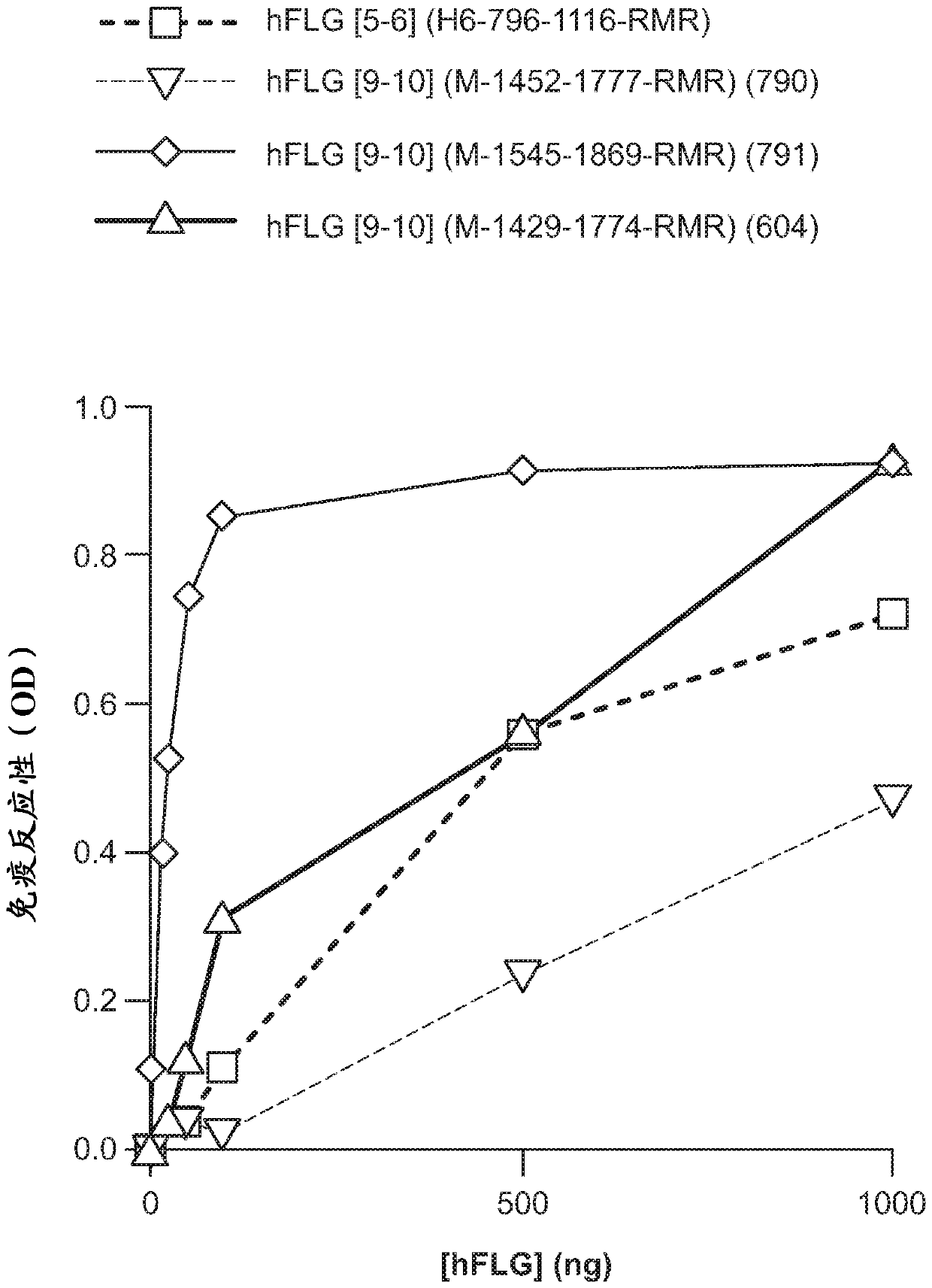
[0167] Data on the localization of purified sGFP and sGFP+RMR will help understand: (i) whether sGFP+RMR penetrates the cuticle; (ii) if so, how deep penetration can be detected; and (iii) Penetration dynamics. Here, 5.0 μg / μL of GFP + / - RMR was applied at time points 0, 2, 6, 12, 18, and 24 h to determine the effect of dose on breakthrough and time. The results showed that GFP was detected as deep as the epidermal-dermal junction within 30 minutes of application.
[0168] Table of cellular compartments and penetration depths of in situ secreted sGFP proteins from SE-GFP reporter and control strains sign
[0169] The goals of this assay are similar to those described above, but with the key difference that the protein is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com