Triazole CYP51-HDAC double-target antifungal compound as well as preparation method and application thereof
A compound, dual-target technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
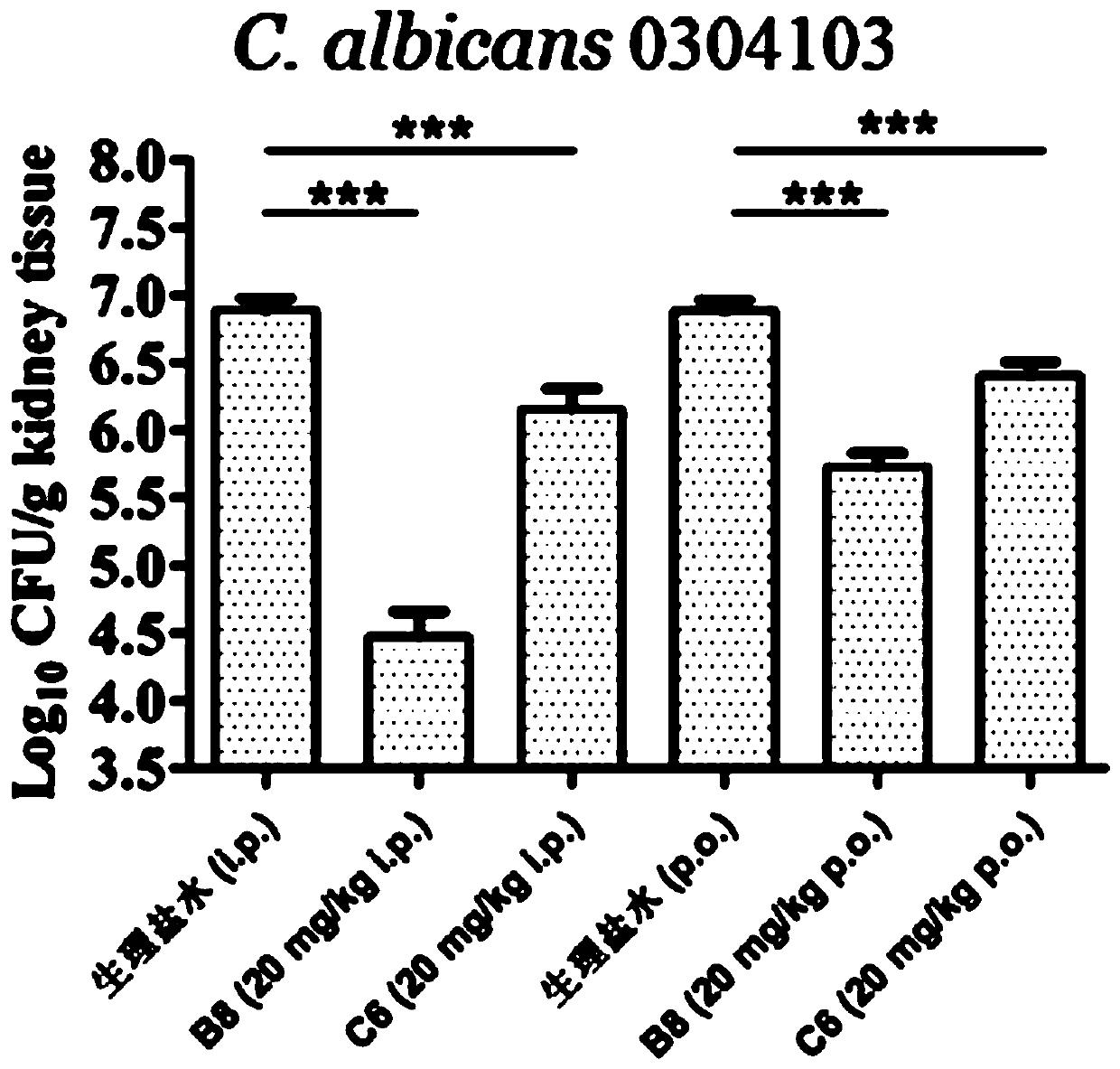
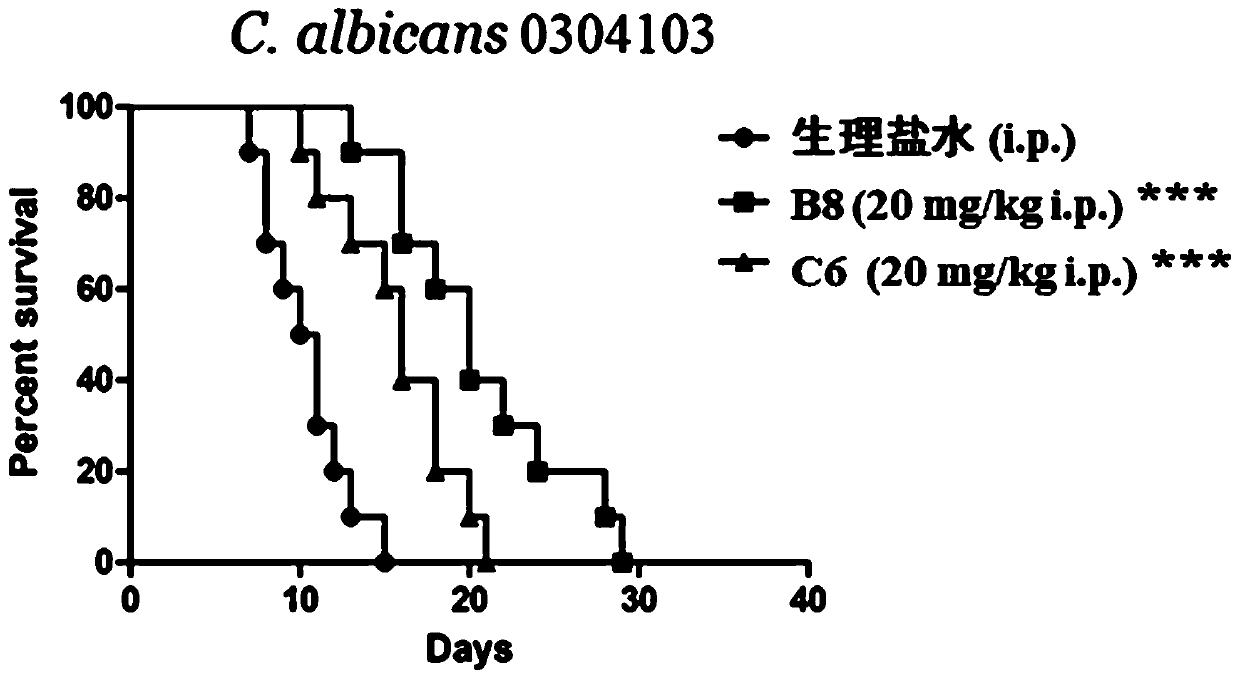
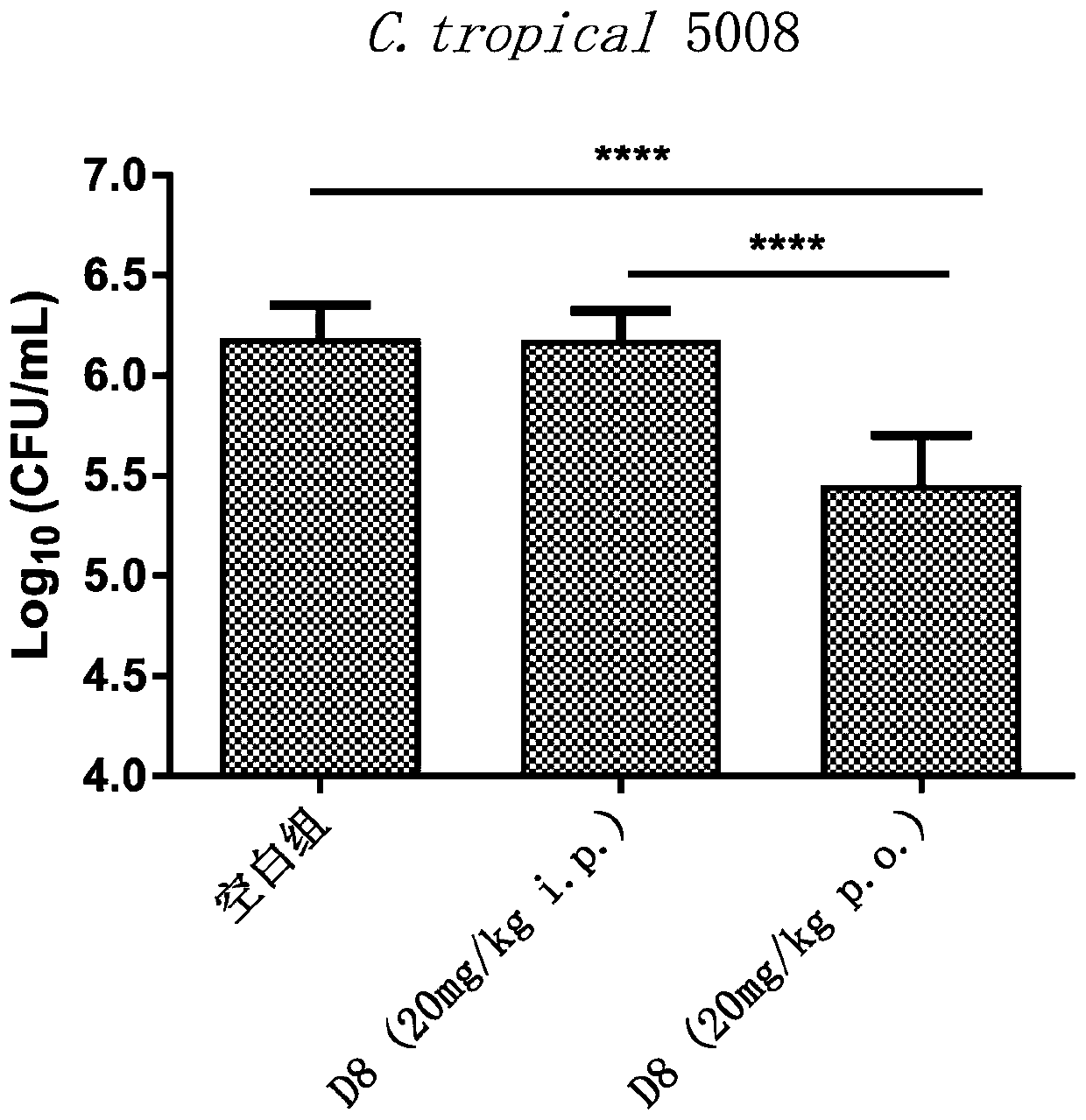
Image
Examples
Embodiment
[0119] Preparation of Compound A:
[0120]
[0121] Reagents and conditions: (a)Br(CH 2 ) mCN, K 2 CO 3 , DMF, 80°C, 12h; (b) (i) HCl, anhydrous MeOH, anhydrous Dioxane rt, 1h; (ii), HCONHNH 2 ,Et 3 N, MeOH, rt, 18h; (c), K 2 CO 3 , DMF, 80℃, 4h; (d), NH 2 OH, KOH, MeOH, rt, 5h.
[0122] The preparation method of compound A1:
[0123] In the first step, 0.5 g of compound 1 (n=0) was dissolved in 5 mL of dry DMF solution, and 0.73 g of reagent Br(CH 2 ) 3 CN, 1.36g K 2 CO 3 , react at 80°C for 12h, filter the filtrate, add 100mL ethyl acetate to dilute and wash three times with saturated aqueous sodium chloride solution, take the ethyl acetate layer, anhydrous Na 2 SO 4 Dry, filter, remove the organic solvent from the filtrate under reduced pressure, and use n-hexane / ethyl acetate (10 / 1, v / v) as the mobile phase to purify the residue by silica gel column chromatography to obtain compound 2 (n is 0, m is 3) .
[0124] In the second step, dissolve 0.5g of compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com