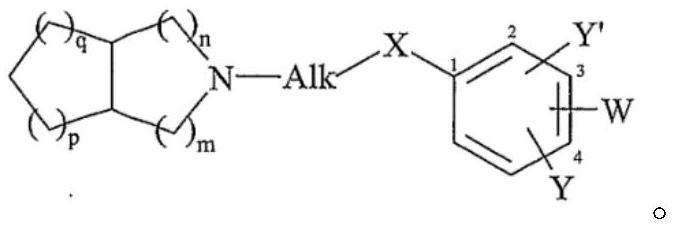
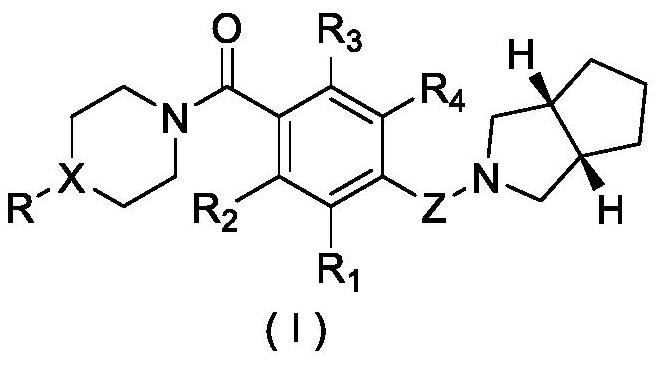
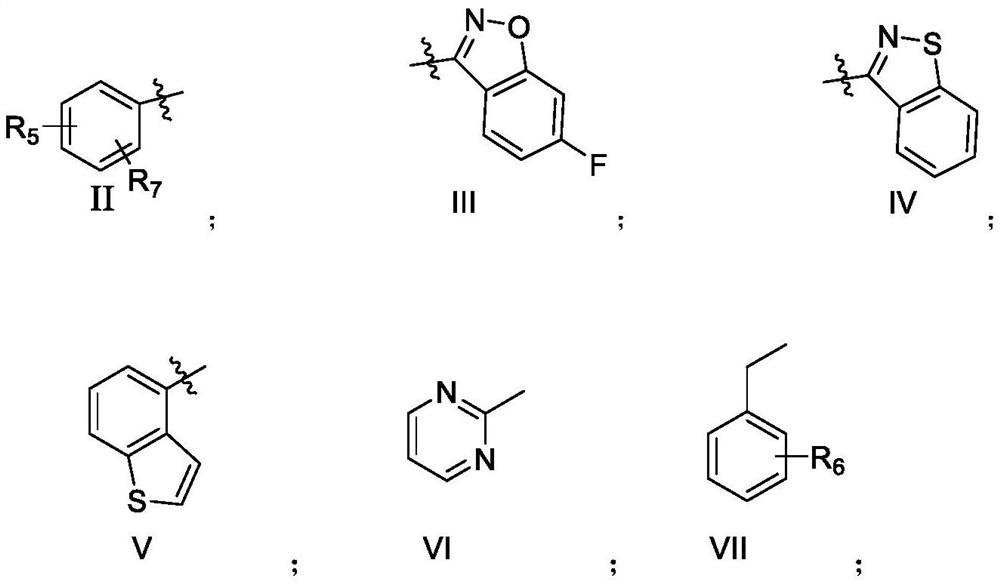
A kind of piperazine benzamide derivatives and application thereof
A technology of benzamide and derivatives, applied in the field of benzamide derivatives and their applications, can solve problems such as adverse reactions, changes, and increased suicide risks, and achieve strong antidepressant activity, high affinity, and safety good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1, (4-(2,3-dichlorophenyl)piperazin-1-yl)(4-(3-(hexahydrocyclopentane[c]pyrrol-2(1H)-yl)propoxy yl)phenyl)methanone (Compound 1)
[0043] Reaction 1
[0044]
[0045] 1) Take 4.6 g of methyl 4-hydroxybenzoate, 9.0 g of 1,3-dibromopropane and 12.4 g of potassium carbonate, add 50 ml of acetone, and heat under reflux for 6 hours. TLC detection, the reaction was completed, cooled to room temperature, evaporated to dryness, added an appropriate amount of dichloromethane, washed with water, separated the aqueous layer, dried the organic layer with anhydrous magnesium sulfate, evaporated the solvent to dryness to obtain a light yellow oil, which was washed with eluent. Petroleum ether:ethyl acetate 5:1, column chromatography gave 7.3 g of white solid, melting point 126-128°C, yield 89.0%.
[0046]2) Take 5.4 g of the first step product, 8.2 g of anhydrous potassium carbonate, 50 ml of acetonitrile, 2.7 g of octahydrocyclopentane[c]pyrrole, heat under reflux for 6...
Embodiment 2
[0050] Example 2, (4-(4-chlorophenyl)piperazin-1-yl)(4-(3-(hexahydrocyclopentane[c]pyrrol-2(1H)-yl)propoxy)benzene yl) ketone (compound 3)
[0051] Substitute 2,3-dichlorophenylpiperazine for 4-chlorophenylpiperazine, and prepare the target compound according to the method of Example 1.
[0052] 1H NMR(400MHz, CDCl3)δ8.25-7.67(m,2H),7.31-6.94(m,4H),6.94-6.37(m,2H),4.08(t,J=12.3Hz,2H),3.59( t,J=8.3Hz,2H),3.16(t,J=8.3Hz,2H),3.00-2.67(m,2H),2.42(t,J=12.2Hz,2H),2.21-1.99(m,2H) ),1.97-1.56(m,8H),1.54-1.25(m,2H).MS(ESI)m / z 468.2([M+H]+).
Embodiment 3
[0053] Example 3, (4-(3-chlorophenyl)piperazin-1-yl)(4-(3-(hexahydrocyclopentane[c]pyrrol-2(1H)-yl)propoxy)benzene yl) ketone (compound 4)
[0054] Substitute 2,3-dichlorophenylpiperazine for 3-chlorophenylpiperazine, and prepare the target compound according to the method of Example 1.
[0055] 1H NMR(400MHz, CDCl3)δ8.06-7.75(m,2H),7.11-6.94(m,2H),6.82-6.77(m,2H),6.66-6.61(m,2H),4.04(t,J =15.1Hz,1H),3.59(t,J=10.3Hz,4H),3.16(t,J=10.2Hz,4H),2.99-2.66(m,2H),2.42(t,J=15.3,2H) ,2.22-2.00(m,2H),1.99-1.12(m,10H).MS(ESI)m / z 468.2([M+H]+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com