A kind of African swine fever virus recombinant antigen and its application
A technology of African swine fever virus and recombinant antigen, which is applied in the field of genetic engineering, can solve problems such as difficult to meet the needs of the grassroots, and achieve the effect of improving specificity, good diagnostic sensitivity and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
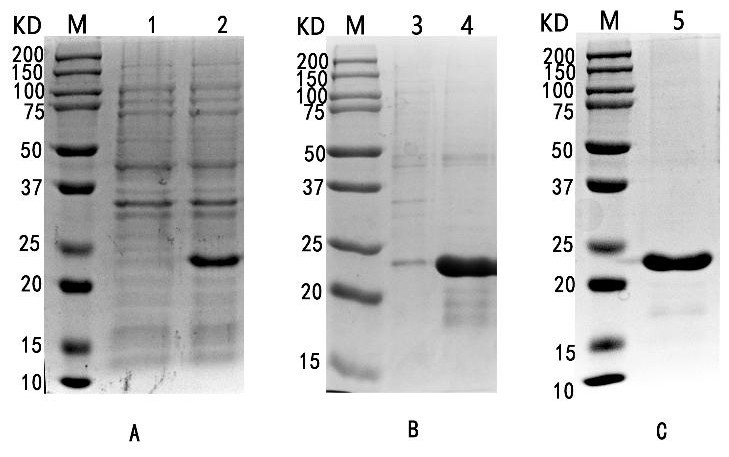
[0040] Example 1 Preparation of m35 protein
[0041] The amino acid sequences of p30 and p54 proteins of African swine fever virus were obtained from GenBank, and their antigenic epitopes were predicted by online software ABCpredPrediction, Scratch, NetCTL and IEDB, and the restriction endonucleases BamH I and EcoR I were selected to introduce flexible cleavage sites. Chain connection sequence, optimized to obtain SEQID NO: 2;
[0042] Send the gene sequence to GenScript to construct plasmid pET-28a(+)-m35;
[0043] 1. Inducible expression of m35 recombinant protein
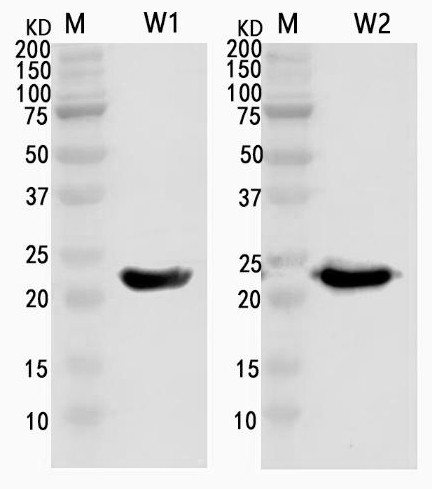
[0044] The pET-28a(+)-m35 vector was transformed into E. coli BL21, spread on a plate, and cultured at 37°C overnight; a single colony was picked, added to 5 mL of LB liquid medium containing Amp, and shaken at 37°C overnight; according to 1: The cultured bacterial liquid was inoculated into 5 mL of LB liquid medium containing Amp resistance at a ratio of 100, and shaken at 37 °C to the bacterial liquid OD. 60...
Embodiment 2
[0060] The establishment of embodiment 2ELISA optimal reaction method
[0061] 1. Determination of optimal antigen coating concentration and optimal antibody dilution
[0062] (1) Dilute the antigen (purified m35) with PBS buffer, use Costar microtiter plate (Costar 42582USA) to coat and detect the diluted antigen, and select the concentration of 1, 0.5, 0.25, 0.125, 0.0625 μg / ml The diluted antigen was kept overnight at 4°C, blocked with 1% BSA at 37°C for 2 hours on the second day, and the liquid was discarded and patted dry.
[0063] (2) Adding samples: Take the ELISA plate coated with African swine fever specific recombinant antigen m35 in step (1), and add 1 / 50, 1 / 100, and 1 / 200 dilutions to the detection well and its duplicate well, respectively. Standard positive serum (serum infected with African swine fever is a positive control) and non-infected negative serum (serum not infected with African swine fever is a negative control), react at 37°C for 60 minutes in the da...
Embodiment 3
[0091] Embodiment 3ELISA kit
[0092] The kit includes: an enzyme-labeled plate coated with antigen standard solution, an HRP-labeled enzyme-labeled secondary antibody and other matching reagents; the enzyme-labeled detection antibody is anti-pig IgG (Sigma SAB3700434-2MG) with HRP-labeled enzyme-labeled secondary antibody );
[0093] The kit also includes sample diluent, washing solution and stop solution;
[0094] The diluent is PBS buffer solution with a pH of 7.4;
[0095] The washing solution is a phosphate buffer containing 0.05% Tween-20;
[0096] The stop solution is 2M sulfuric acid solution;
[0097] Enzyme substrate solution chromogenic solution TMB (Surmodics TMBW-1000-01);
[0098] The positive control is the positive serum of pigs artificially infected with African swine fever in the laboratory;
[0099] The negative control was the serum of pig control group not infected with African swine fever in the laboratory.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com