Flavanol-menthane heterozygote as well as pharmaceutical composition, preparation method and application thereof
A flavanol and menthane technology, applied in the field of flavanol-menthane hybrids, can solve the problem of no compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
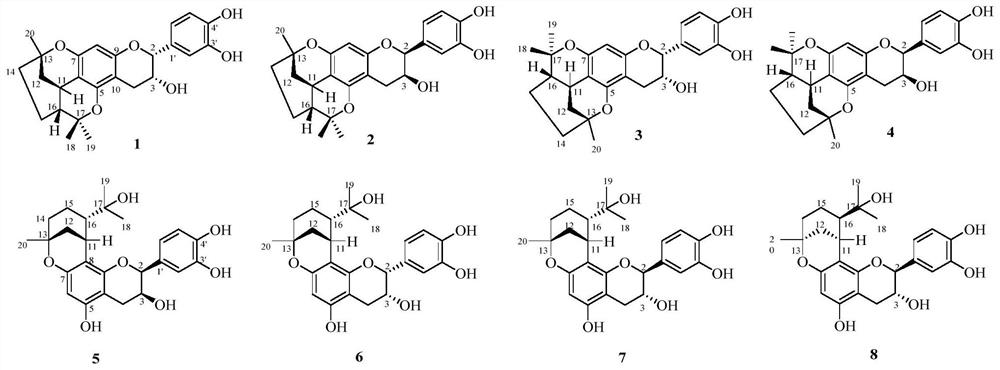
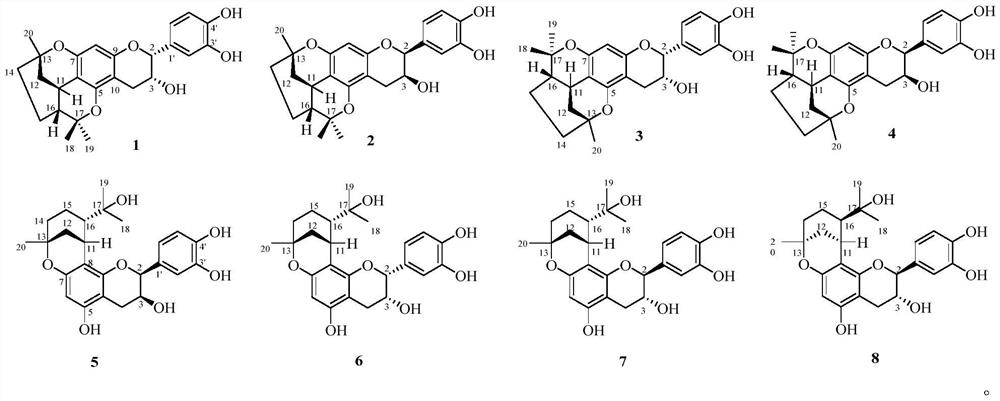
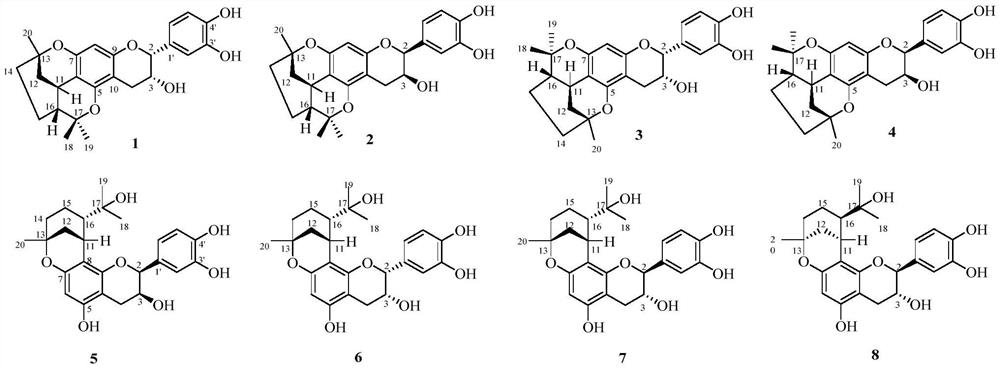
[0029] Preparation of Compounds 1-8:
[0030] The dried fruit of Cao Guo was crushed and extracted three times with 50% ethanol under reflux for 2 hours each time. The ethanol extracts were combined, and the ethanol was recovered under reduced pressure to obtain the extract. The extract was dispersed in water and extracted with ethyl acetate, then concentrated to the ethyl acetate extract. Then the ethyl acetate fraction (Fr.A) was subjected to silica gel column chromatography, eluting with methanol-chloroform (0:100, 5:95, 10:90, 20:80 and 40:60, v / v) Seven fractions of Fr.A-1~Fr.A-7 were obtained by gradient elution of reagents. Fraction Fr.A-6 was subjected to MCI CHP 20P gel column chromatography (methanol-water, 40:60, 60:40, 80:20 and 100:0) to obtain four subfractions Fr.A-6-1~Fr.A-6-4. Fr.A-6-2 was subjected to silica gel column chromatography (MeOH-CHCl 3 , 2:98) to obtain three subfractions Fr.A-6-2-1 ~ Fr.A-6-2-2. Fr.A-6-2-2 (2.2g) was purified by Sephadex LH-20...
Embodiment 2
[0127] PTP1B and α-glucosidase inhibitory activity
[0128] 1 Materials and methods
[0129] 1.1 Materials
[0130] α-glucosidase (Sigma Aldrich, St.Louis, MO, USA); phosphate buffer (≥99%, Meilun Bio, Dalian); p-nitrophenyl-α-D-glucopyranose (≥99%) %, Yuanye Biology, Shanghai); acarbose (≥98%, Bayer Pharmaceuticals, Beijing); PTP1B (protein tyrosine phosphatase) was purchased from Sino Biological (Wayne, PA, USA); suramin sodium was purchased from From ACROS (New Jersey USA)
[0131] 1.2 Instruments
[0132] Flex Station 3 desktop multifunctional microplate reader (Bio-RAD 680, USA); analytical balance (AG135, Metler Toledo, China); thermostat box (DHP-9082, Shanghai).
[0133] 1.3 Experimental process
[0134] PTP1B inhibitory activity was performed according to the inventors' previous studies. Briefly, the working buffer (workingbuffer, WB) was composed of 3-(N-morpholino)propanesulfonic acid (MOPS, 722.02mg), dithiothreitol (DTT, 30mg), EDTA (25.7mg), It is prepared...
preparation Embodiment 1~8
[0146] In the following preparation examples, conventional reagents are selected, and preparations are prepared according to existing conventional methods. This example only reflects that at least one of the compounds 1 to 8 of the present invention can be prepared into different preparations. Specific reagents and The operation is not specifically limited:
[0147] 1. After dissolving at least one of the compounds 1 to 8 prepared in Example 1 with DMSO, add water for injection according to a conventional method, fine filter, potting and sterilizing to make an injection, and the concentration of the injection is 0.5 ~5 mg / mL.
[0148] 2. After dissolving at least one of the compounds 1 to 8 prepared in Example 1 with DMSO, dissolve it in sterile water for injection, stir to dissolve it, filter it with a sterile suction filter funnel, and then sterilize it. filtered, divided into ampoules, freeze-dried at low temperature and sealed aseptically to obtain a powder injection.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap