Anti-vamp2 antibody for inhibiting snare complex and use thereof
An antibody and antigen technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, application, drug combination, etc., can solve problems such as insufficient antibody research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Screening of VAMP2 scFv Antibody
[0069] 1. Biopanning
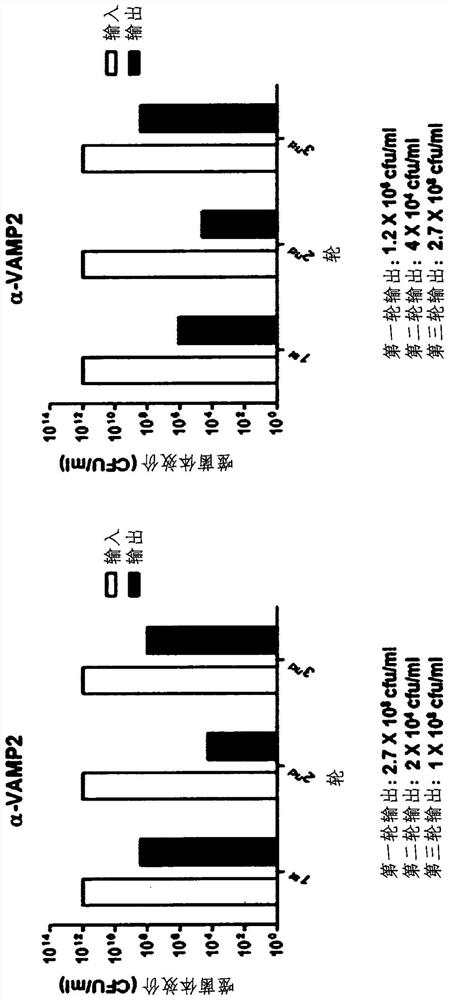
[0070] Use a 7.6 x 10 9 The diversity of the OPAL library was biopanned. 4 μg of VAMP2 antigen was immobilized on epoxy magnetic beads and reacted with input phage. Output titers were measured by the elution of phage reactive with the antigen. Both input and output titers are measured each time to gain information about the biopanning and confirm that it is functioning properly. For each time, use ≥4×10 12Input phage in cfu / ml of phage. exist figure 1 The results of sequential panning up to round 1, round 2 and round 3 are shown in . Get 1×10 respectively 8 cfu / ml and 2.7×10 8 The output of VAMP2 in cfu / ml ( figure 1 ). Therefore, it was confirmed that the biopanning proceeded normally.
[0071] 2. ELISA analysis
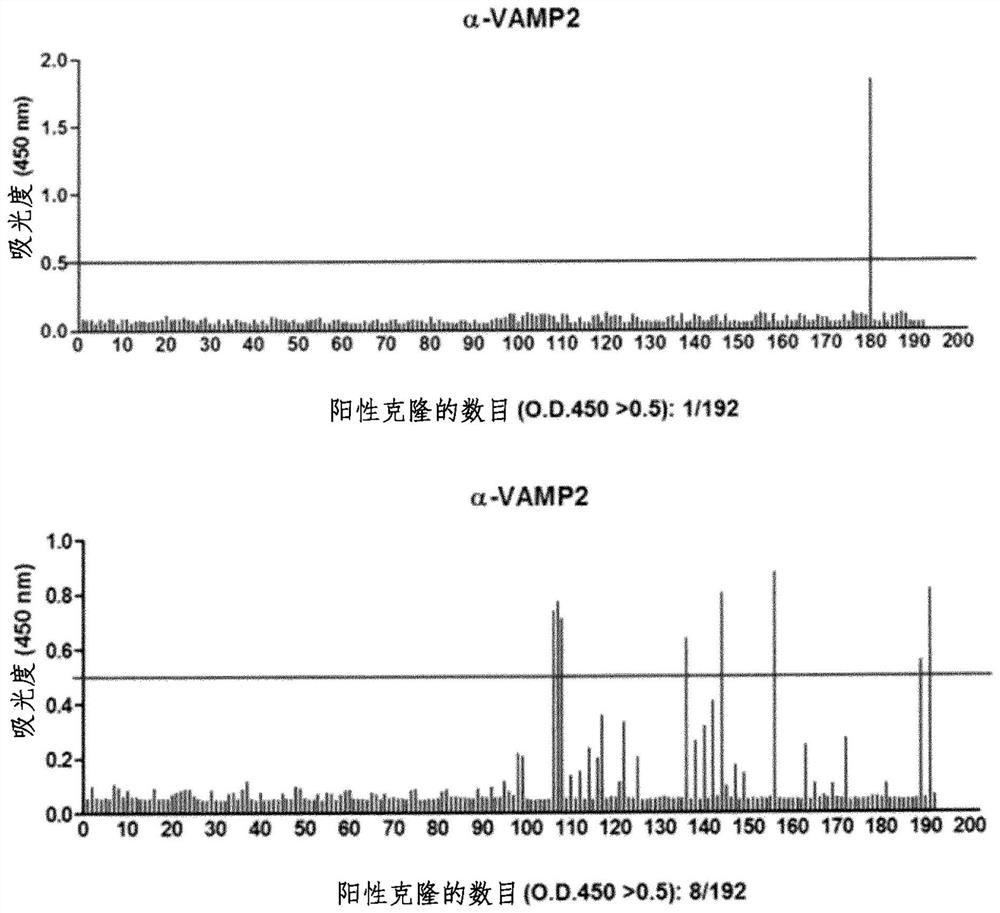
[0072] ELISA analysis was performed to screen antibodies with high sensitivity and specificity. The obtained phages were infected with E. coli and streaked on LB plates suppleme...
Embodiment 2
[0073] Example 2 VAMP2 scFv antibody sequence analysis
[0074] Nine positive clones for VAMP2 screened by ELISA analysis were sequenced, whereby a single clone was screened. Sequencing is necessary because there may be duplicate clones among previously screened positive clones. As a result of sequence analysis, it was confirmed that all 9 VAMP2s were the same clone (Table 1).
[0075] [Table 1]
[0076] CDR1 CDR2 CDR3 CDR1 CDR2 CDR3 VAMP1 L TGSSSNIGSNNVT SDSH GSWDYSLSA H NYSMS AIYSOGSSI KYRSSKHTPLPSYSNAMDV
Embodiment 3
[0077] Example 3 Preparation of cell-permeable anti-VAMP2 scFv
[0078] 1. Preparation of TAT-VAMP2 scFv construct
[0079] By using one of the previously selected scFvs as a primer, a PCR product inserted with a restriction site and a TAT was produced. 30 µl of each PCR product was treated with 4 µl of buffer 3.1, 1 µl of Sal1, 1 µl of Xho1, and 4 µl of distilled water, reacted at 37°C for 1 hour, and then isolated DNA to obtain inserts to be inserted into vectors. Transform the pET28a(+) vector into DH5α competent cells, then streak on LB plates supplemented with kanamycin, incubate at 37°C for 16 hours, and inoculate the colonies on Namycin in LB medium and incubate at 37 °C for 16 hr. The next day, isolate the vector using a mini-prep kit, and treat 30 µl of the vector with 4 µl of buffer 3.1, 1 µl of Sal1, 1 µl of Xho1, 1 µl of CIP, and 3 µl of distilled water to react at 37°C for 1 hour, Then purify. Concentrations of purified vector and insert were measured by nanod...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap