A kind of highly active catalyst for ammonia synthesis and preparation method thereof
A high-activity catalyst, a technology for ammonia synthesis, applied in the preparation/separation of ammonia, chemical instruments and methods, catalysts for physical/chemical processes, etc., can solve problems such as affecting the catalytic effect of active metals, achieve good industrial application prospects, good ammonia Effects of synthetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
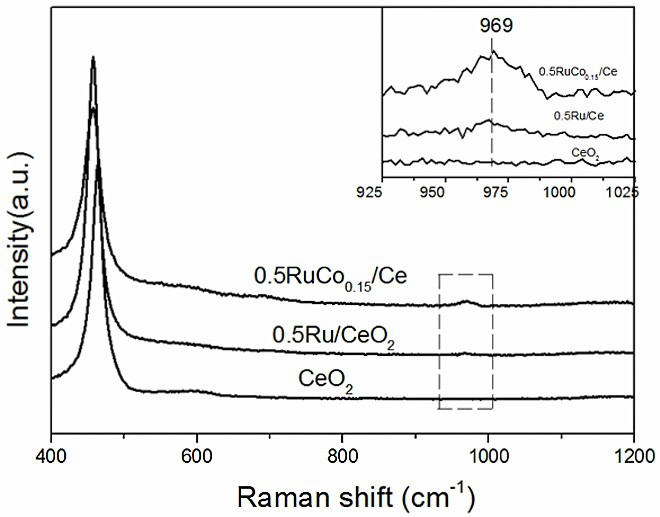
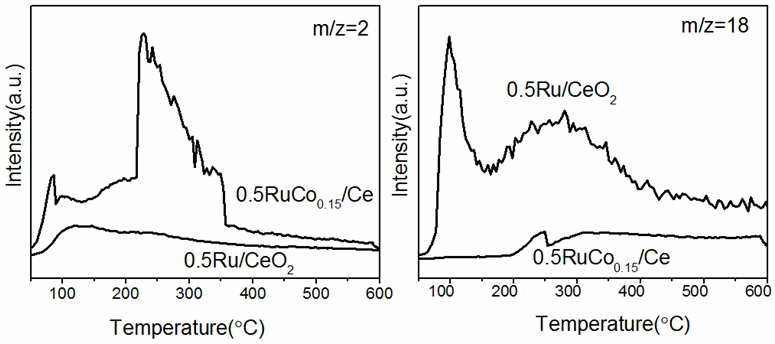
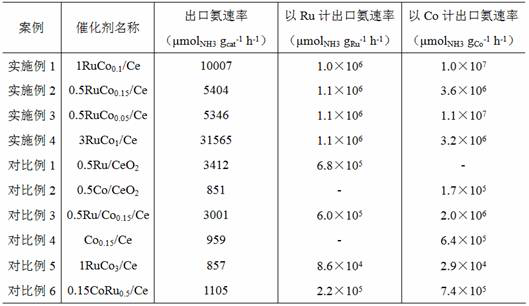
Image
Examples
Embodiment 1
[0024] Take 2.538 g of cerium acetate and 23 mg of cobalt nitrate mixed and dissolved in 20 mL of deionized water to form a mixed solution, take 38.4 g of NaOH dissolved in 140 mL of distilled water to form an alkaline solution; then mix and stir the two solutions for 1 hour, at 160 After hydrothermal reaction at ℃ for 6 hours, a solid solution was obtained; the solid solution was washed to neutrality, dried at 100 ℃ overnight, and calcined at 400 ℃ for 1 hour, and the obtained sample was impregnated with 10 g / L carbonyl ruthenium alcohol solution 3 hours; then in H2 volume concentration of 10% 2 -Ar mixed gas, after reduction at 600 °C for 4 hours, the catalyst 1RuCo 0.1 / Ce, calculated based on the mass of cerium oxide, the addition amounts of ruthenium metal and cobalt metal in the catalyst are 1% and 0.1%, respectively.
Embodiment 2
[0026] Take 1.736 g of cerium nitrate and 2.6 mg of cobalt chloride and mix and dissolve in 30 mL of deionized water to form a mixed solution, and dissolve 20 g of KOH in 60 mL of distilled water to form an alkaline solution; then mix and stir the two solutions for 0.5 hours, and then A solid solution was obtained after a hydrothermal reaction at 100 °C for 24 hours; the solid solution was washed to neutrality, dried at 80 °C for 2 hours, and calcined at 550 °C for 2 hours, and the obtained sample was treated with 15 g / L nitrosyl nitrate Ruthenium alcohol solution was soaked for 2 hours; then the catalyst 0.5RuCo was obtained after reduction with pure hydrogen at 500 °C for 2 hours 0.15 / Ce, based on the mass of cerium oxide, the addition amounts of ruthenium metal and cobalt metal in the catalyst are 0.5% and 0.15%, respectively.
Embodiment 3
[0028] Mix and dissolve 3.472 g cerium nitrate and 2.3 mg cobalt nitrate in 40 mL deionized water to form a mixed solution, and dissolve 38.4 g NaOH in 140 mL distilled water to form an alkaline solution; The solid solution was obtained after hydrothermal reaction for 24 hours; the solid solution was washed to neutrality, dried at 80 °C for 2 hours, and calcined at 550 °C for 2 hours, and the obtained sample was impregnated with 5 g / L ruthenium chloride alcohol solution 2 hours; then in 10%H 2 -3.3%N 2 Catalyst 0.5RuCo 0.05 / Ce, based on the mass of cerium oxide, the addition amounts of ruthenium metal and cobalt metal in the catalyst are 0.5% and 0.05%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com