Cell-mediated exosome delivery
A cell and receptor technology, applied in the field of delivery of protein therapeutics and RNA therapeutics, can solve the problems of preventing the complete activation of CART cells, the inapplicability of adverse event mitigation strategies, and increasing the druggable targets of SynNotch technology and similar platforms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0054]Construct design and cloning: ORFs are usually produced synthetically and cloned into mammalian expression vectors, for example, pSF-CAG-Amp. Briefly, the synthesized DNA and vector plasmid were digested with Notl and Sall enzymes according to the manufacturer's instructions. According to the manufacturer's instructions, use T4 ligase to join the restricted, purified DNA fragments together. Successful ligation events were selected by bacterial transformation on plates supplemented with ampicillin. According to the manufacturer's instructions, the plasmid for transfection was generated by "maxi-prep".
[0055]Cell culture and transfection: Depending on the experimental design and measurement, non-viral or viral transfection of the genetically engineered cells in this article. Transfection and transduction are carried out in various cell culture systems, including but not limited to 2D cell culture, shaking incubators, various forms of bioreactors, hollow fiber bioreactors, wave ba...
example 1
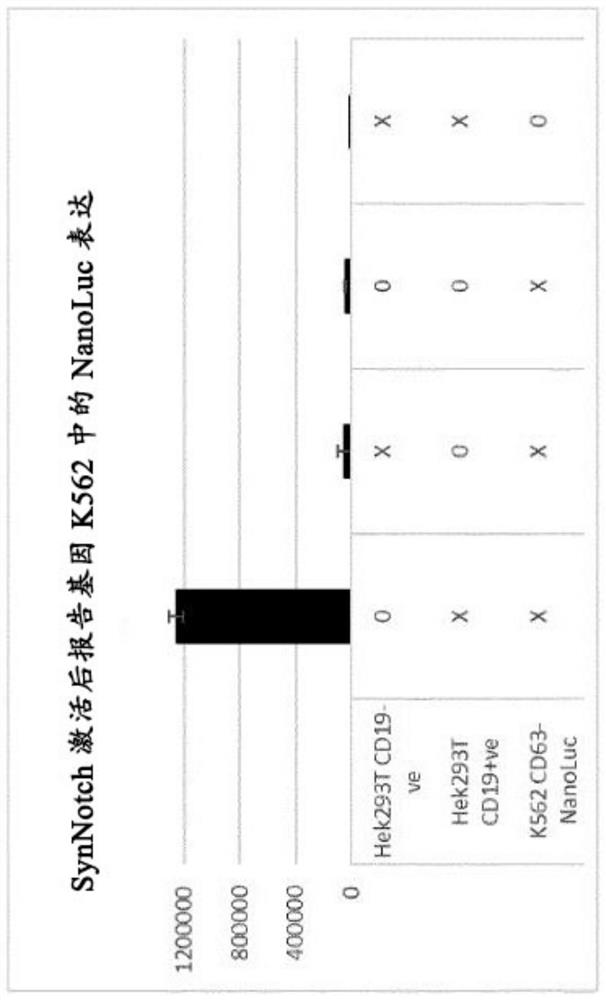
[0057]Example 1. Through CD19-dependent chimeric Notch polypeptide activation, CD63-NanoLuc is expressed in K562 cells.
[0058]K562 cells are engineered to express CD19-sensing chimeric Notch polypeptides, which activate and release intracellular transcription factors that induce CD63-NanoLuc expression. In addition, two HEK293T cell lines were produced, one with stable CD19 expression and one without. Each HEK293T cell and reporter gene chimeric Notch polypeptide K562 cells were seeded in a 24-well plate. Forty-eight hours later, EVs were harvested from the conditioned medium and NanoLuc activity was measured. The NanoLuc activity measured in samples without CD19 is negligible, while in the presence of CD19, the reporter gene K562 cells express CD63-Nanoluc in EVs. This data indicates that only through the presence of CD19 and its interaction with the chimeric Notch polypeptide on K562 cells, CD63-Nanoluc can be expressed and loaded into EVs. A similar experiment was performed, but t...
example 2
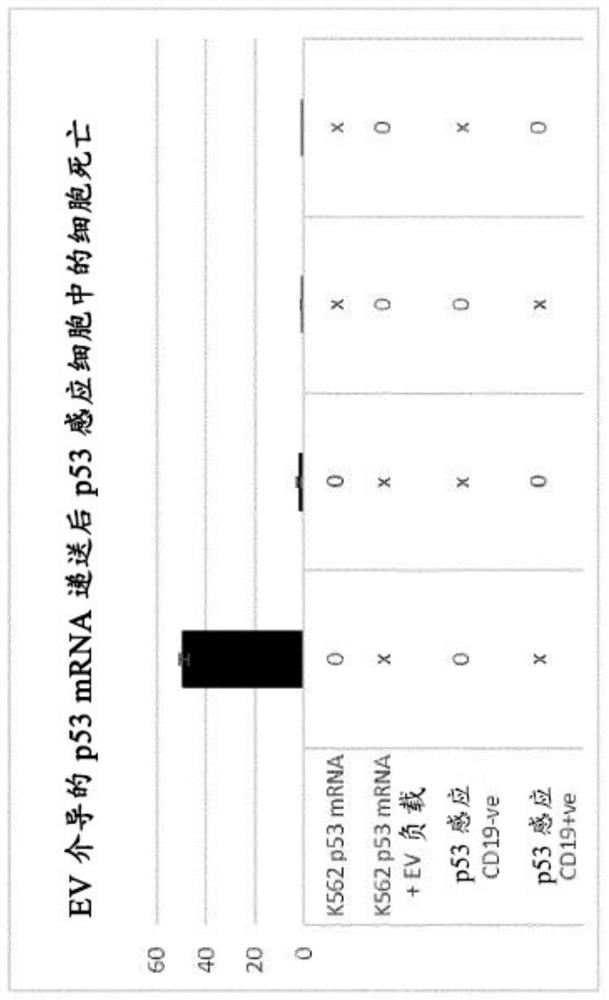
[0059]Example 2. After uptake of CD63-intein-Cre from stable K562 chimeric Notch polypeptide cells, GFP expression in Cre recombinase-responsive MDA cells.
[0060] K562 cells are engineered to express CD19-sensing chimeric Notch polypeptides, which activate the expression of CD63-intein-Cre or soluble Cre recombinase. CD63-Intein-Cre is a previously described system that allows Cre recombinase to be loaded into EVs and finally released from any exosomal protein. In addition, the MDA-MB-231 Cre responsive cell line was engineered to stably express CD19. Through the activity of Cre recombinase, the expression of GFP in reporter gene cells is permanently activated. The combination of MDA-Lox+ve or -Lox-ve and K562-CD63-intein-Cre or -Cre were inoculated in a 24-well plate. Forty-eight hours later, GFP+ve cells were counted for the remaining MDA population (MDA cells constitutively express red fluorescent protein, which turns green by Cre activity). In the presence of CD19 and subsequent...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap