Recombinant vector and expression and purification method of heat-labile UNG fusion protein
A recombinant vector, expression and purification technology, applied in the field of expression and purification of heat-labile UNG fusion protein, can solve the problems of wrong conformation of expressed protein, low expression amount, degradation of UNG enzyme, and miscellaneous protein.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
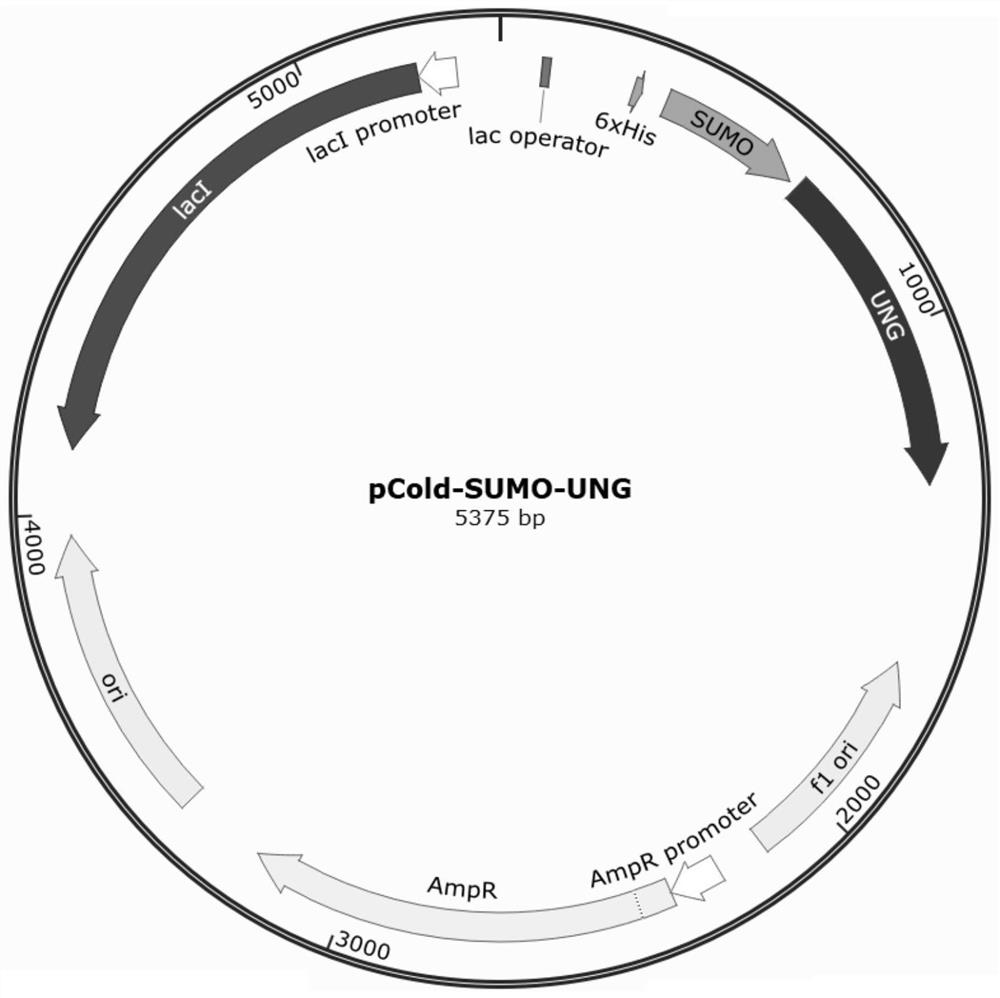
[0056] Embodiment 1: Construction of pCold-SUMO-rCod UNG vector
[0057] The rCOD UNG gene sequence was amplified by PCR, using SEQ ID NO: 1 and SEQ ID NO: 2 as primers, using SEQ ID NO: 3 (synthesized by Shanghai Sangon Bioengineering Co., Ltd.) as a template, and using PFX as a DNA polymerase to amplify The amplification system was 50ul, and the amplification conditions were 98°C, 2min; 98°C, 15s; 64°C, 15s; 72°C, 15s; 72°C, 2min, a total of 35 cycles, and PCR product 1 was obtained.
[0058] Electrophoresis was performed on 1.2% (w / v) agarose gel, and the target band was excised for gel recovery to obtain product 1.
[0059] Digest 3ug of pCold-SUMO plasmid with 20U each of PstI-HF and KpnI-HF, double-digested vector pCold-SUMO (SEQ ID NO:4), react in water bath at 37°C for 4 hours, cut out the target strip after 1.2% agarose gel electrophoresis The band was recovered by gel to obtain product 2, and the digested product was transformed into TOP10 competent cells (purchased...
Embodiment 2
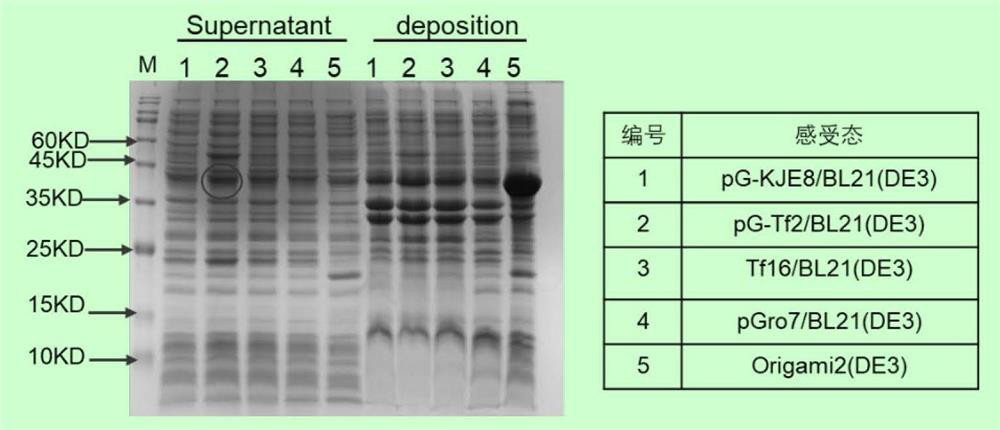
[0065] Example 2: Small expression of 6XHis-SUMO-rCod-UNG
[0066] The strain preparation of the pCold-6XHis-SUMO-rCod-UNG expression vector: take 25ng of the pCold-6XHis-SUMO-rCod-UNG expression vector obtained in Example 1, and add it to 100 μL of 5 kinds of plasmids containing different molecular chaperones (see image 3 ) BL21 (DE3) competent cells (purchased from Shanghai Nearshore Technology Co., Ltd.), ice-bathed for 30 minutes, then heat-shocked at 42°C for 90 seconds, inserted back on ice for 2 minutes, and coated with ampicillin (30 μg / ml) and chlorine Mycin (10 μg / ml) double-resistant LB medium, 37 ° C constant temperature incubator inverted overnight.
Embodiment 3
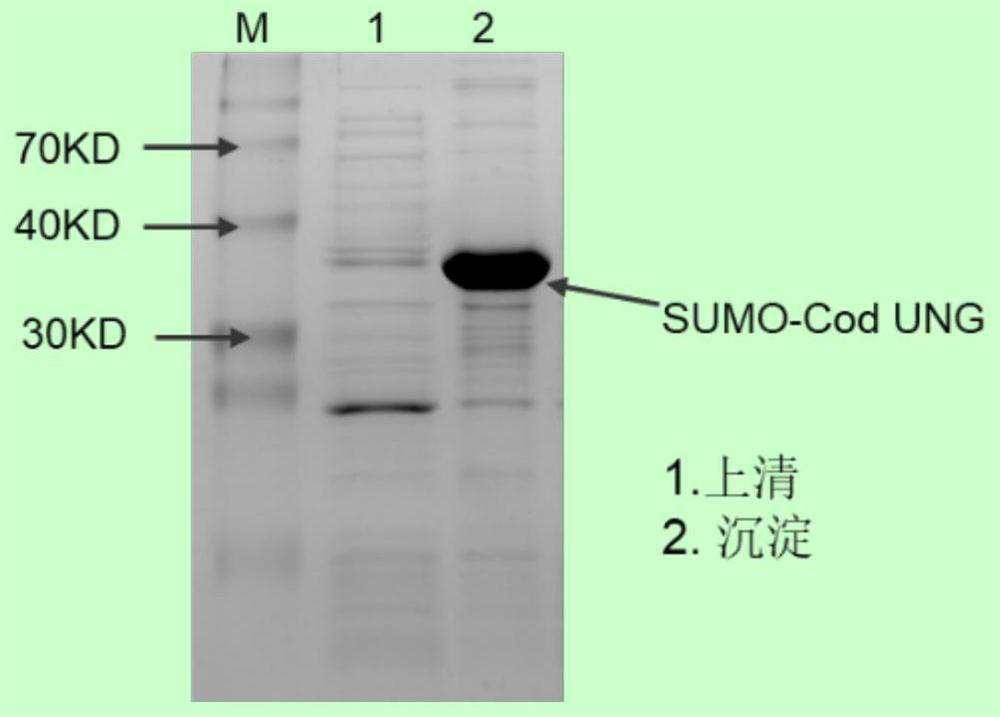
[0075] Example 3: Purification of 6XHis-SUMO-rCod-UNG
[0076] ① Large-scale induction of protein expression: Take the pCold-6XHis-SUMO-rCod-UNG expression strain obtained in Example 2, cultivate it overnight to obtain seed liquid, and transfer it to 1L ampicillin (30 μg / ml) and chlorine-containing solution at a ratio of 1:100. In liquid LB of mycin (10μg / ml), culture on a shaker at 37°C at 200rpm until OD600 reaches 0.5-0.7, take it out and cool it on ice for 15min, then add IPTG with a final concentration of 0.25mM, place at 15°C at 200rpm Shaker cultured for 16h to induce protein expression.
[0077] ② Lyse the cells: collect the cells by centrifugation for the first time, place the collected cells at -80°C, and freeze them for more than 30 minutes. Take out the frozen cells and resuspend the cells with pre-cooled 50ml His-tag Binding buffer (induced expression bacteria Liquid volume: Histag Binding buffer volume = 20:1). The cells were sonicated in an ice-water bath at 5...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap