Recombinant human thyrotropin injection
A technology for thyrotropin and thyroxine, which is applied in the field of biomedicine, can solve the problems of difficulty in ensuring biological activity, difficulty in thyrotropin storage and increase the cost of medication for patients, and achieves the effect of high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0259] 1.1 Materials and Instruments
[0260] The reagent information and instrument information used in the examples of the present application are shown in Table 1 and Table 2 below, respectively.
[0261] Table 1 Reagent information
[0262] Reagent name factory Item No. Disodium hydrogen phosphate dodecahydrate Sigma 04273-1kg Sodium dihydrogen phosphate monohydrate Merck 106349 9025 Sodium chloride Merck 137017 5000 Mannitol Sigma M8429-500G Poloxamer 188 Merck 1.37065.1000 L-methionine Sigma M5308-100g Tween 20 Merck 817061 1000
[0263] Table 2 Instrument information
[0264] equipment name factory model Nucleic acid protein detector Thermo Nanodrop 2000 Desktop centrifuge Beckman Coulter Allegra TM X-12R-Centrifuge
-80℃ ultra-low temperature refrigerator Haier DW-87L 386 Drug stability test box Shanghai Yiheng Scientific Instrument Co., Ltd...
Embodiment 2
[0284] Experiment 2: Effects of different concentrations of sodium chloride and mannitol on the storage effect of liquid preparations
[0285] This example explores the effects of different concentrations of sodium chloride and mannitol on the storage effect of the thyrotropin liquid preparation. The prescription composition of each test group in Test 2 is shown in Table 4. The liquid formulation was subjected to a 60-day accelerated stability test at 25±2°C to simulate the impact of long-term storage conditions under extreme conditions on the sample.
[0286] The prescription composition of each test group of table 4 test two
[0287]
[0288] Note: The protein concentration indicates the protein concentration of recombinant human thyrotropin, and the PB buffer indicates phosphate buffer.
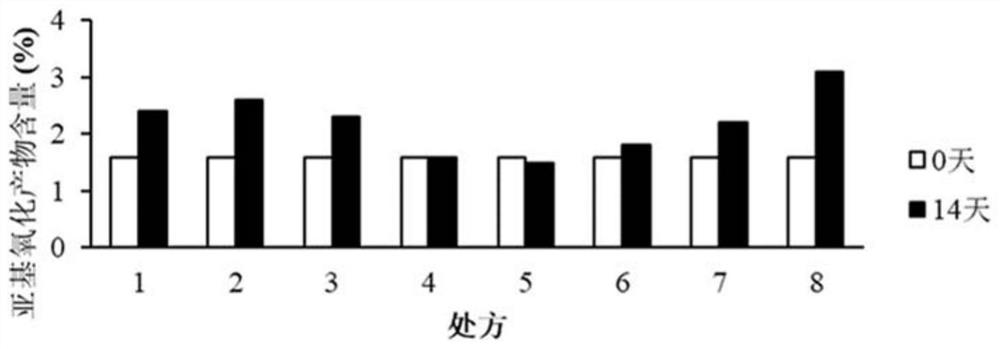
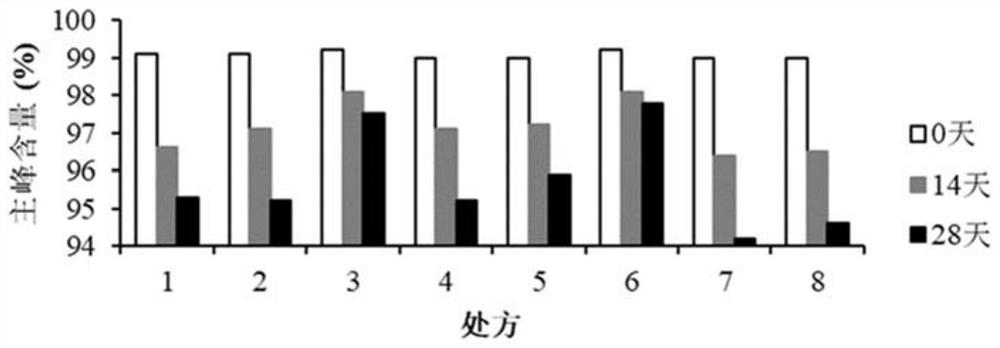
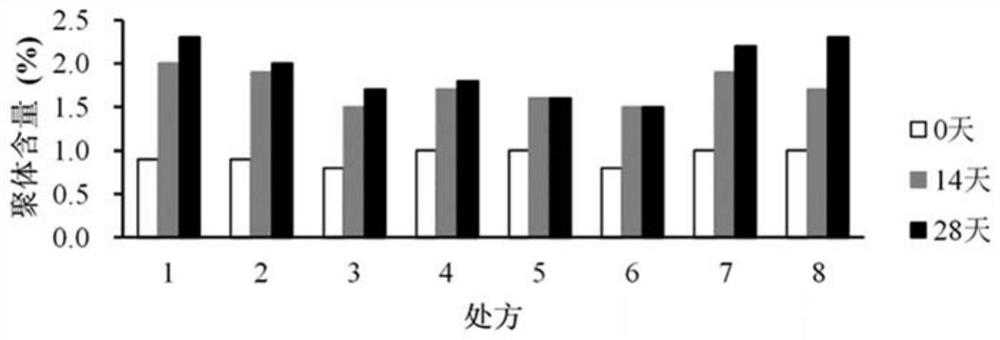
[0289] Detection results and analysis of the content of subunit oxidation products
[0290] Such as Figure 6 As shown, using the samples of 12 prescriptions in Experiment 2, the de...
Embodiment 3
[0294] Experiment 3: Effect of different methionine concentrations on the storage effect of liquid preparations
[0295] This example explores the effects of different methionine concentrations on the storage effect of thyrotropin liquid preparations. The prescription composition of each test group in Test 3 is shown in Table 5. The liquid formulation was subjected to a 60-day accelerated stability test at 25±2°C to simulate the impact of long-term storage conditions under extreme conditions on the sample.
[0296] The prescription composition of each test group of table 5 test three
[0297] prescription protein concentration PB buffer Mannitol Sodium chloride L-methionine pH 1 0.9mg / mL 20mM 30mg / mL 2mg / mL 0.1mg / mL 7.0 2 0.9mg / mL 20mM 30mg / mL 2mg / mL 0.2mg / mL 7.0 3 0.9mg / mL 20mM 30mg / mL 2mg / mL 1.5mg / mL 7.0
[0298] Note: The protein concentration indicates the protein concentration of recombinant human th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap