Anti-platelet membrane glycoprotein IB ALPHA humanized antibody and application thereof
A technology for humanizing antibodies and glycoproteins, which can be used in the field of biomedicine and can solve problems such as evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Humanization of murine antibody NIT-B1
[0105] by murine antibodies NIT-A1 and NIT-B1 (described in U.S. Patent Serial No. 8323652 and deposited at the International Depository of Canada on October 7, 2008, respectively, with accession number 071008-01 (clone NIT A1) And 071008-02 (clone NIT B1)) sequencing found that the murine antibody NIT-A1 contains a heavy chain sequence SEQ ID NO: 1 (including CDR1 sequence SEQ ID NO: 3, CDR2 sequence SEQ ID NO: 4 and CDR3 sequence SEQ ID NO:5) and a light chain sequence of SEQ ID NO:11 (including CDR1 sequence of SEQ ID NO:13, CDR2 sequence of SEQ ID NO:14 and CDR3 sequence of SEQ ID NO:15). The murine antibody NIT-B1 contains a heavy chain sequence of SEQ ID NO: 6 (including CDR1 sequence of SEQ ID NO: 8, CDR2 sequence of SEQ ID NO: 9 and CDR3 sequence of SEQ ID NO: 10) and a light chain sequence of SEQ ID NO : 16 (containing CDR1 sequence SEQ ID NO: 18, CDR2 sequence SEQ ID NO: 19 and CDR3 sequence SEQ ID NO: 20). ...
Embodiment 2
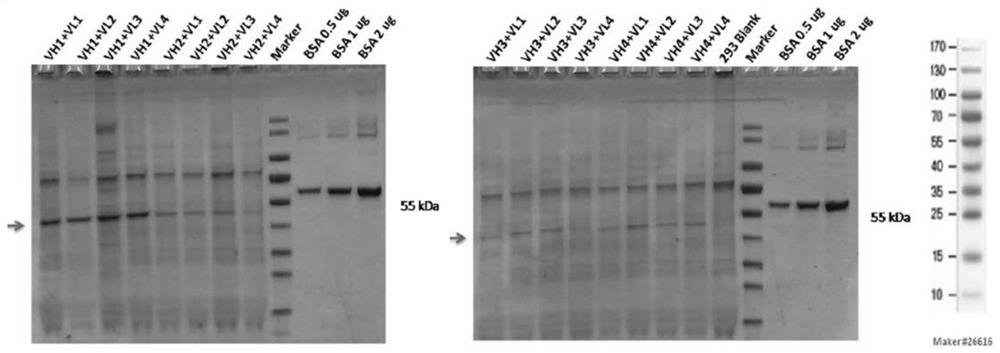
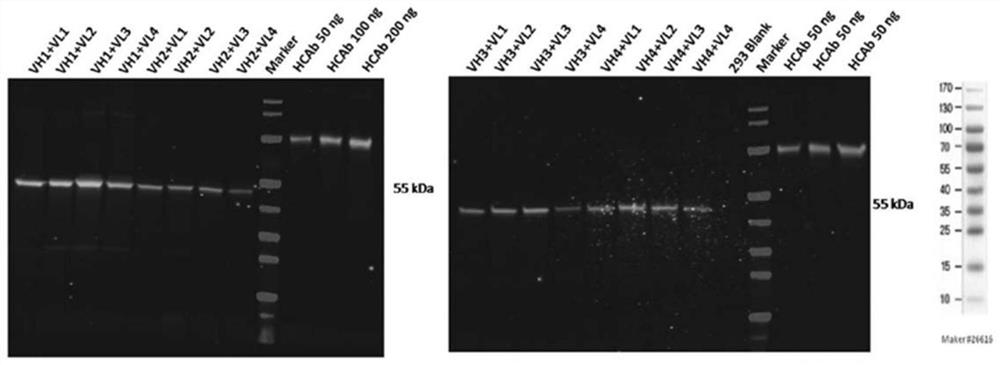
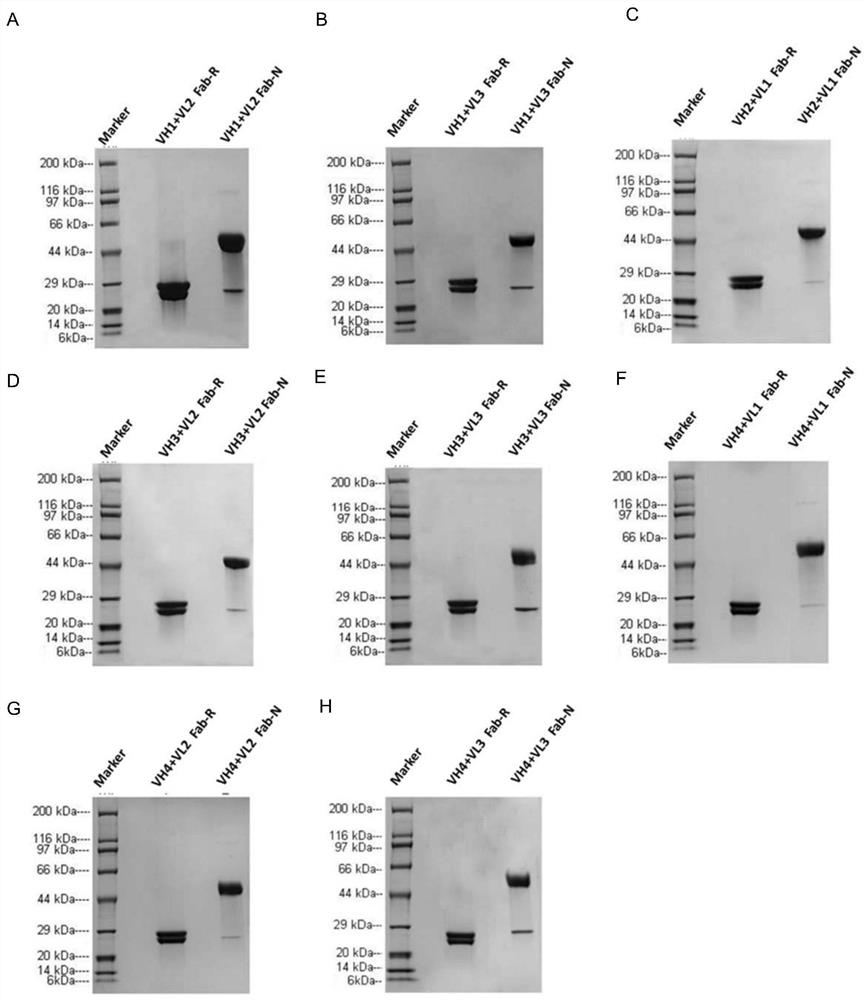
[0107] Example 2 Identification of Humanized Anti-GPIbα Antibody
[0108] Using the CDR grafting method (Safdari et al., 2013), four human heavy chain variable regions (VH1 sequence SEQ ID NO: 35, VH2 sequence SEQ ID NO: 42, VH3 sequence SEQ ID NO: 49 and VH4 sequence SEQ ID NO: 56) and four human light chain variable regions (VL1 sequence SEQ ID NO: 63, VL2 sequence SEQ ID NO: 70, VL3 sequence SEQ ID NO: 77 and VL4 sequence SEQ ID NO: 84) were synthesized. The selection of sequences was based on the homology ranking of human CDR region framework sequences annotated in NCBI database.
[0109] Briefly, the variable domain sequences of the parental antibodies were searched against the database of human germline immunoglobulins using NCBI Immunoglobulin Sequence Blast (http: / / www.ncbi.nlm.nih.gov / projects / igblast / ) . For the heavy and light chains, four different human acceptor sequences (ie, human variable domains with high homology to the parental antibody) were selected, res...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap