TREATMENT OF SKIN DISEASES OR DISORDERS BY DELIVERY OF ANTI-OSMRbeta ANTIBODY
An antibody and disease technology, applied in skin diseases, antibody medical components, antibodies, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
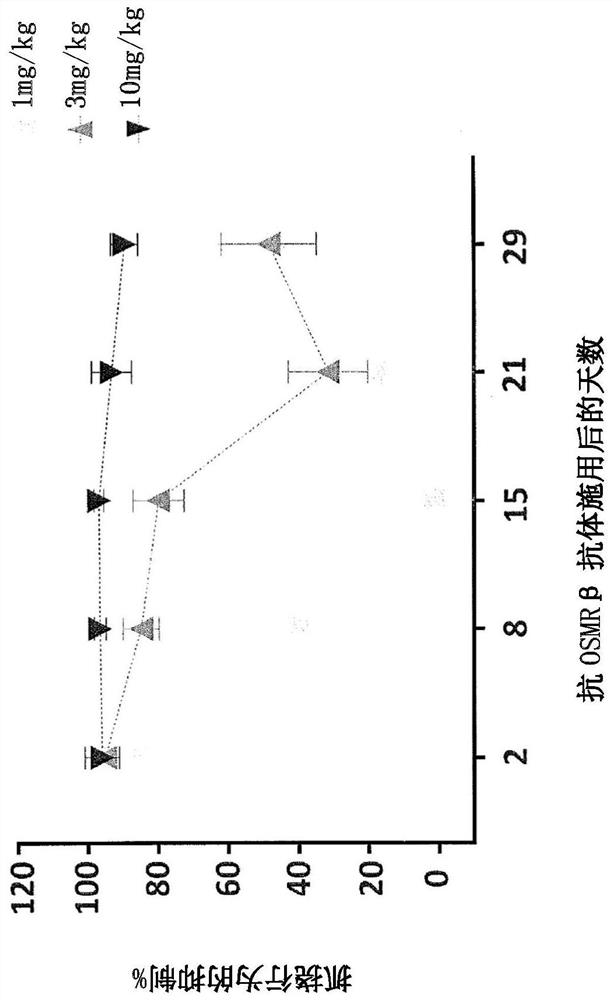
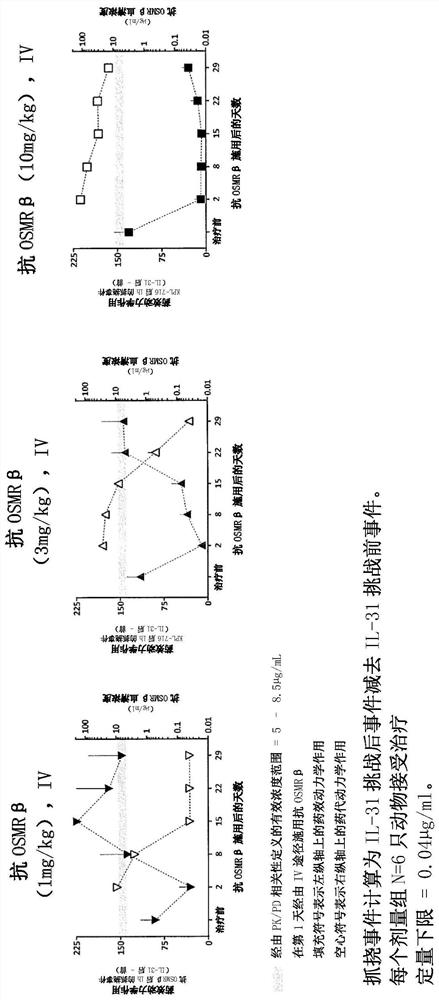
[0376] Example 1: Effect of anti-OSMRβ antibody on cynomolgus monkeys
[0377] The study in this example was designed to evaluate the single-dose pharmacokinetics and efficacy of anti-OSMRβ antibodies following intravenous (IV) administration in male cynomolgus monkeys. A previous study was performed to determine the dose level of human IL-31 that produced the most consistent and strongest scratching response in male cynomolgus monkeys after intradermal administration. The selected dose level was 3 μg / kg human IL-31, which is a supraphysiological level of the IL-31 cytokine.
[0378] experimental design
[0379] animal choice
[0380] Male cynomolgus monkeys were selected from SNBL USA Livestock. Selected animals were physically examined by a veterinarian. In addition, conduct a behavioral assessment prior to the start of the study to exclude animals that may be overgroomed or have pre-existing skin conditions or alopecia. Only animals that met basic health criteria and w...
Embodiment 2
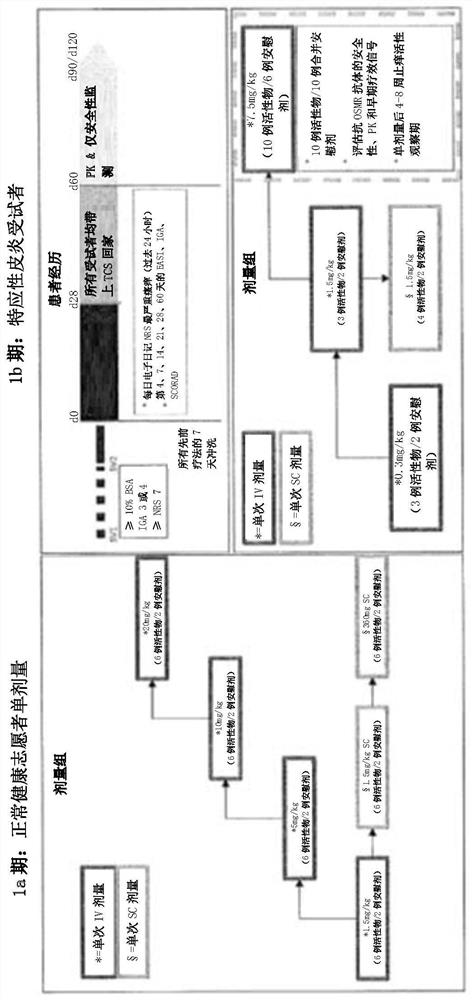
[0403] Example 2: Anti-OSMRβ antibody treatment of atopic dermatitis
[0404] The study in this example was designed to evaluate the safety, tolerability, PK and immunogenicity of anti-OSMRβ antibody in patients with atopic dermatitis. The study also included exploratory studies of pharmacogenetics and the impact of anti-OSMRβ antibodies on clinical efficacy assessments, gene expression and PD measures.
[0405] Research design
[0406] Anti-OSMRβ antibodies were administered intravenously (IV) to subjects with moderate to severe atopic dermatitis experiencing moderate to severe pruritus. In addition, an anti-OSMRβ antibody was administered subcutaneously (SC) to a group of subjects with moderate to severe atopic dermatitis who experienced moderate to severe pruritus.
[0407] Subjects were entered into one of seven groups as described below. After confirmation of eligibility, subjects were randomized to receive anti-OSMRβ antibody or placebo. In six groups, anti-OSMRβ ant...
Embodiment 3
[0442] Example 3: Treatment of Uremic Pruritus Using Anti-OSMRβ Antibody
[0443] The study in this example was designed to evaluate the safety, tolerability, PK and immunogenicity of anti-OSMRβ antibodies in hemodialysis subjects with uremic pruritus. The study also included exploratory studies of pharmacogenetics and the impact of anti-OSMRβ antibody on clinical effect assessment, gene expression and PD measures.
[0444] Research design
[0445] Anti-OSMRβ antibodies were administered intravenously (IV) to hemodialysis subjects with uremic pruritus.
[0446] Subjects entered a treatment group. After confirmation of eligibility, subjects were randomized to receive either 5 mg / kg or 10 mg / kg of anti-OSMRβ antibody or placebo on Day 0 (the day before a routinely planned hemodialysis session).
[0447] After dosing, subjects must be confined to the clinical research unit for at least 2 days of safety monitoring and intensive PK sampling. PK samples were collected at pre-spe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com