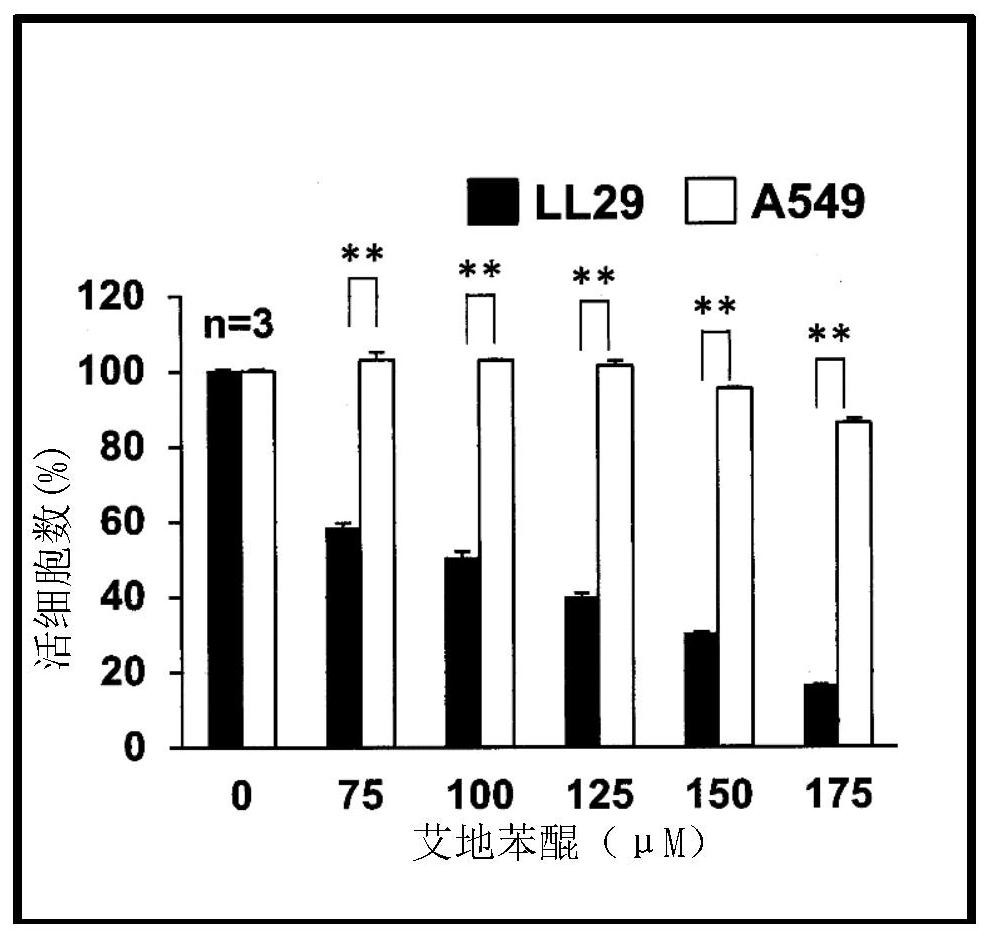
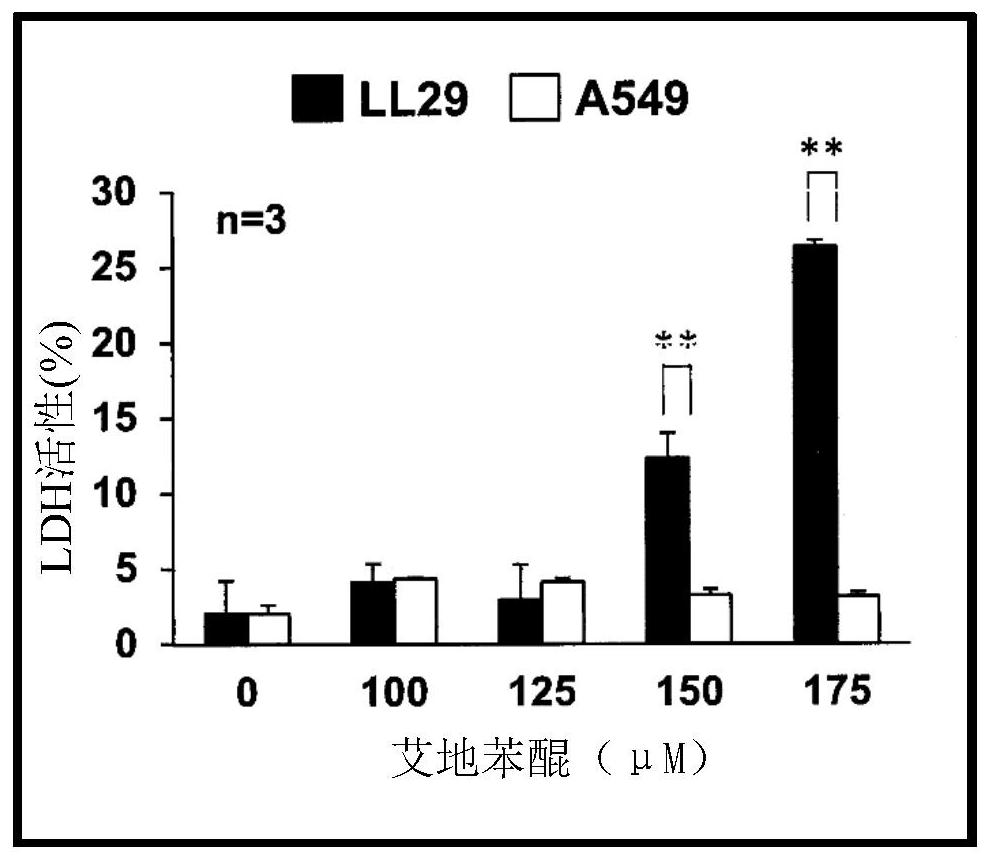
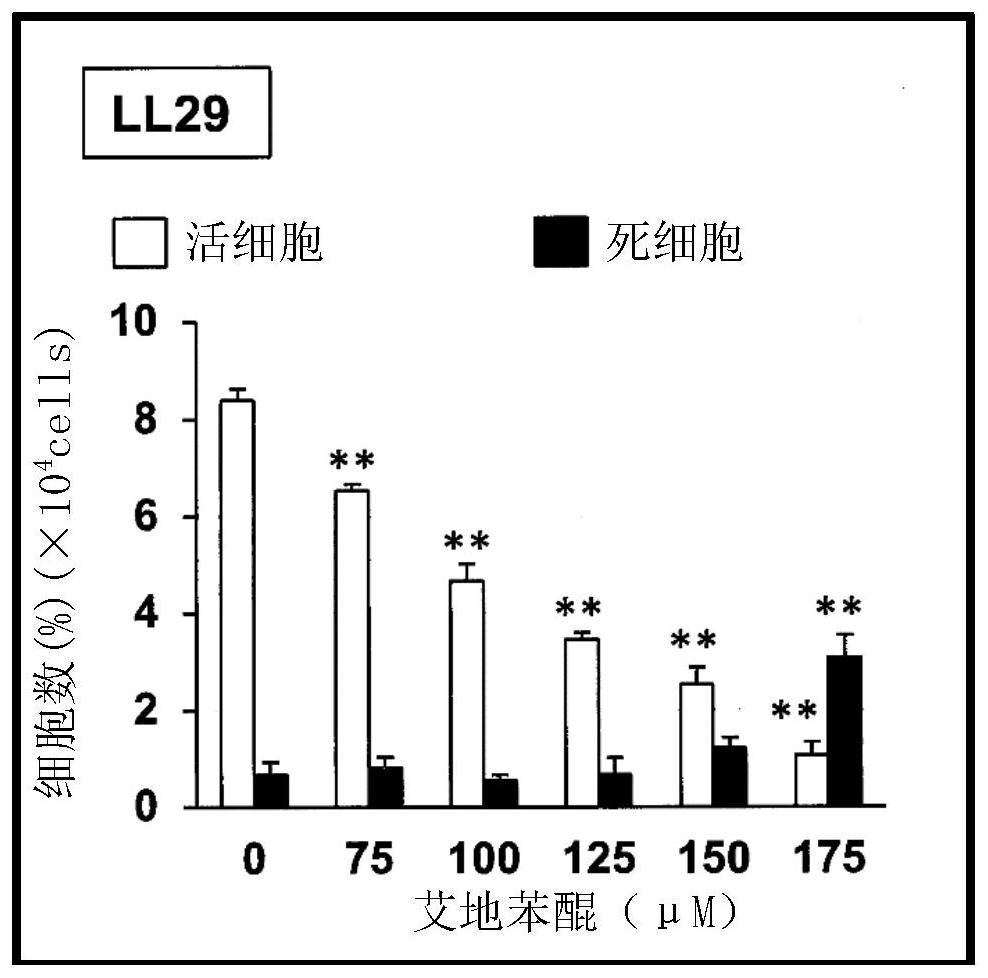
Pharmaceutical composition for treating fibrosis
A pharmaceutical composition and compound technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, drug delivery, etc., can solve problems such as ambiguity, high frequency, and serious side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0125] a.Experimental method
[0126] (1) Administration of bleomycin (BLM), idebenone, tolperisone and eperisone
[0127] For male ICR mice under normal feeding, BLM was administered through the respiratory tract on the first day to make BLM lung injury model mice.
[0128] Pre-administration: From the first day to the seventh day, idebenone was administered through the respiratory tract once a day. On the first day, idebenone was administered through the respiratory tract 1 hour before BLM administration.
[0129] Subsequent administration: From the 10th day to the 18th day, idebenone was administered through the respiratory tract once a day. Both drugs were suspended in 0.9% NaCl before use.
[0130] Post-administration: From the 10th day to the 19th day, tolperone was administered once a day. Both drugs were suspended in 0.9% NaCl before use.
[0131] Post-administration: Eperisone was administered once a day from the 10th day to the 19th day. Both drugs were suspend...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com