Oral pharmaceutical composition of teriparatide or abaloparatide
A technology of teriparatide and composition, applied in the field of oral pharmaceutical composition of teriparatide or abalotide, can solve the problems of poor patient compliance, changing the route of administration of somatostatin and its analogs, etc.
Pending Publication Date: 2021-06-18
SUZHOU LANDING BIOPHARM CO LTD +1
View PDF2 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] Neither teriparatide nor abalotropide can be taken orally, which leads to poor patient compliance. Th
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
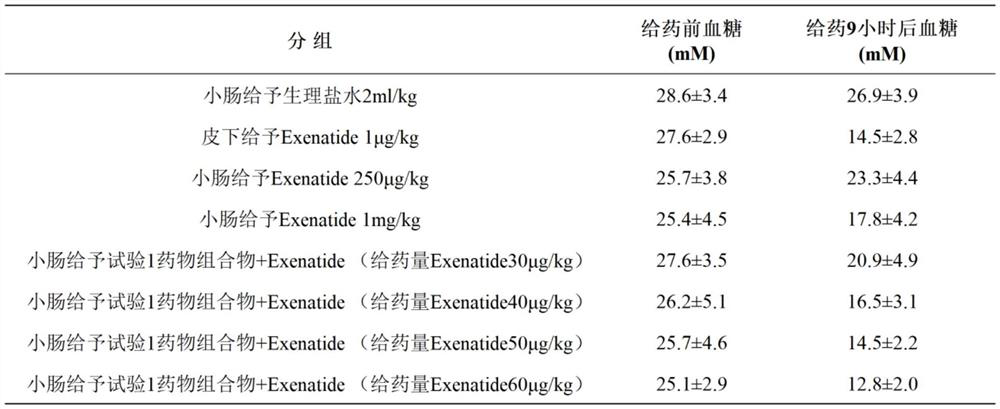
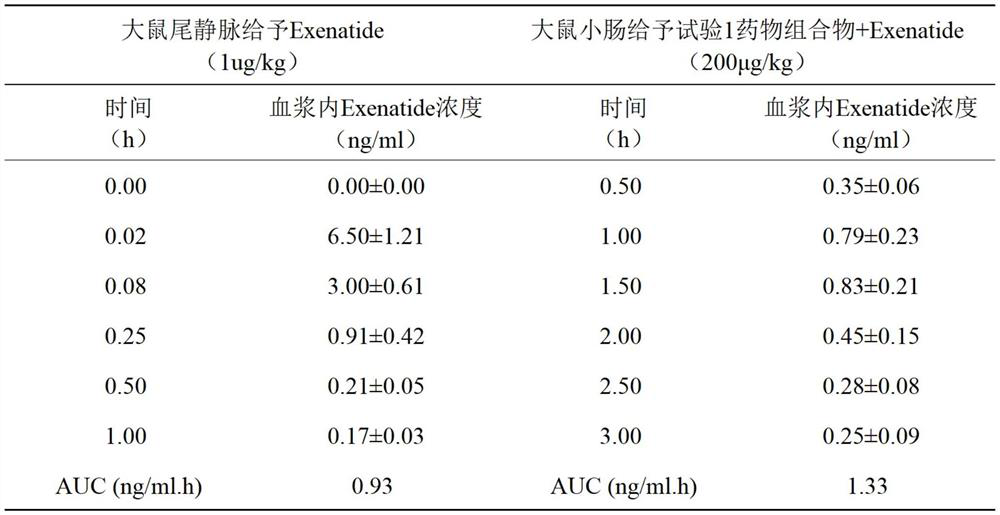
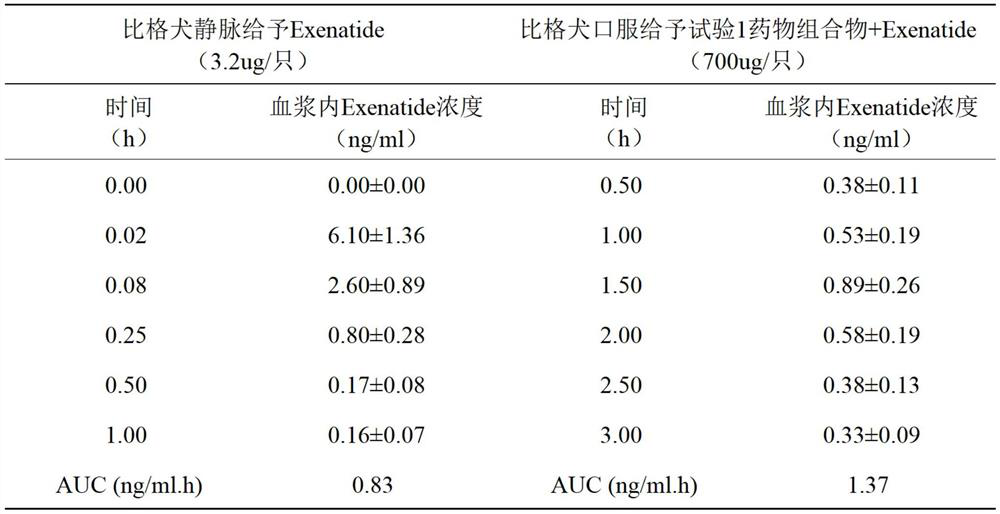
The invention belongs to the field of biological medicine, and particularly relates to an oral pharmaceutical composition of teriparatide or abaloparatide. The pharmaceutical composition is prepared from teriparatide or abaloparatide and a pharmaceutical composition for promoting small intestine absorption, wherein the pharmaceutical composition for promoting small intestine absorption consists of lauryl sodium sulfate, chitosan and sodium citrate. According to the pharmaceutical composition for promoting small intestine absorption, a composite adjuvant is prepared, and after the composite adjuvant is combined with the teriparatide or abaloparatide, the absorption of the effective component in the small intestine can be improved.
Description
technical field [0001] The invention belongs to the technical field of biomedicine, and in particular relates to an oral pharmaceutical composition of teriparatide or abalolotide. Background technique [0002] Teriparatide is a synthetic polypeptide hormone, which is a 1-34 amino acid fragment of human parathyroid hormone PTH, which is the biologically active N-terminal region of endogenous parathyroid hormone PTH containing 84 amino acids. The immunological and biological properties of this drug are identical to those of endogenous parathyroid hormone PTH and bovine parathyroid hormone PTH (bPTH). Teriparatide stimulates bone formation and resorption, reduces the incidence of fractures in postmenopausal women, and, depending on the mode of administration, increases or decreases bone mineral density. Continuous infusions result in sustained increases in PTH concentrations and thus produce greater bone resorption than daily injections that result in only transient increases ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K38/29A61K9/00A61K47/20A61K47/36A61K47/12A61P19/10
CPCA61K38/29A61K9/0053A61K47/20A61K47/36A61K47/12A61P19/10
Inventor 张菁金文波
Owner SUZHOU LANDING BIOPHARM CO LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com