Application of cinobufagin in medicine for treating acute myelogenous leukemia
A technology for cinobufin-based and myeloid leukemia, which is applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
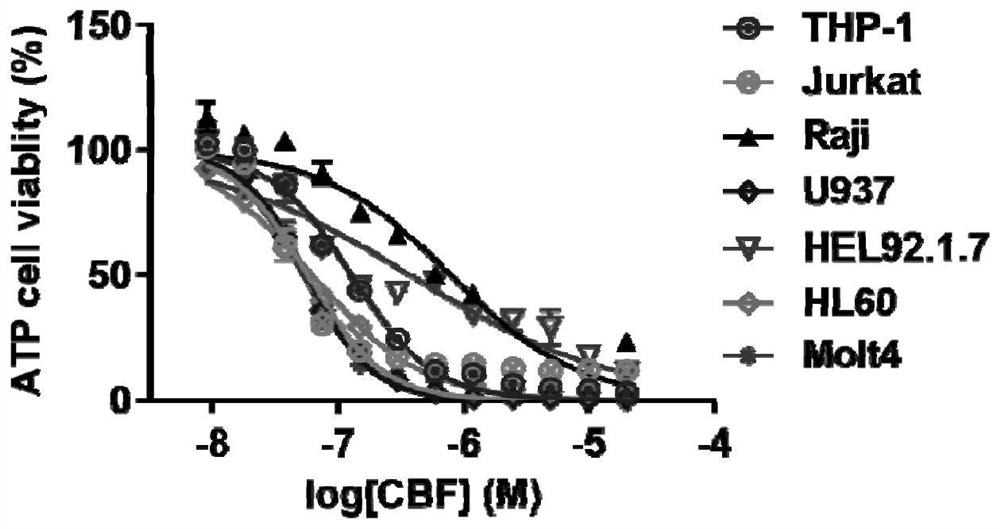
[0026] AML cell lines THP-1 and HL60 and other leukemia cell lines Jurkat, Raji, U937, HEL92.1.7 or Molt4 were cultured, and 3×10 4 Cells were seeded in a 96-well plate at a final concentration of 100.00 μM, 50.00 μM, 25.00 μM, 12.50 μM, 6.20 μM, 3.10 μM, 1.50 μM, 0.80 μM, 0.40 μM, 0.20 μM, 0.10 μM, 0.05 μM and 0 μM The cinobufaction base was treated for 18 hours, and then the CellTiter-Lumi TM The requirements for the use of the luminescent cell viability detection kit are to detect cell viability.
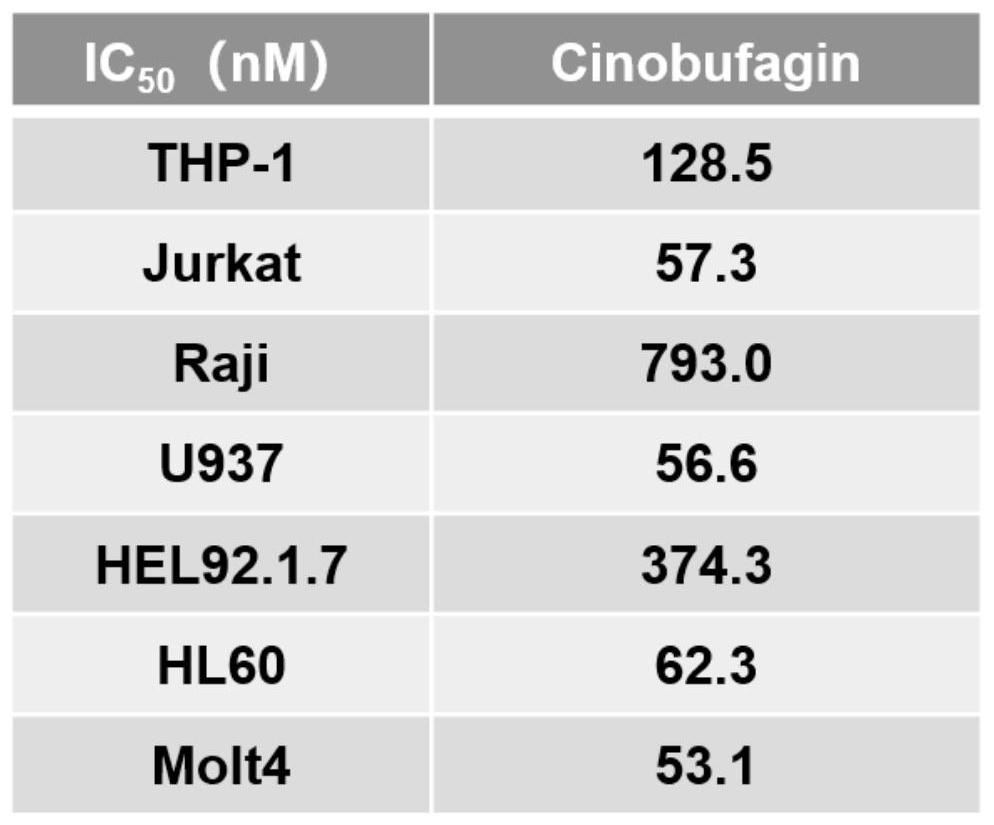
[0027] After detection, the 0 μM group was regarded as the control group, and the cell viability was counted as 100%, and the relative cell viability of other groups was calculated. Then use GraphPad software, take the logarithmic value of the cinobufaction base concentration as the abscissa, and take the relative cell viability as the ordinate, to obtain the relationship between the concentration of the cinobufin base and the cell viability (such as figure 1 ).
[0028] The i...
experiment example 2
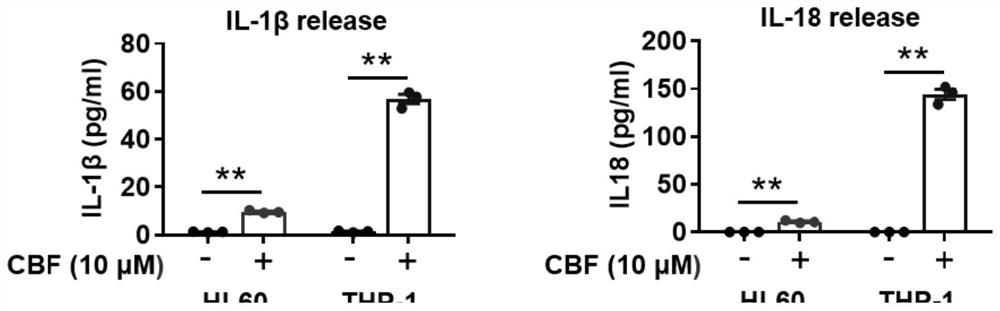
[0031] 2105 cells of AML cell lines THP-1 and HL60 were inoculated in 48-well plates, treated with 1 μM cinobufaction base for 18 hours, the culture supernatant was collected by centrifugation, and the contents of IL1β and IL18 in the supernatant were analyzed by ELISA , the data results are as image 3 shown. It was confirmed that when cinobufaction base induces the death of THP-1 and HL60 cells, it will secrete inflammatory factors IL1β and IL18, which can effectively regulate the tumor immune microenvironment and activate the anti-tumor immune response mediated by T cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com