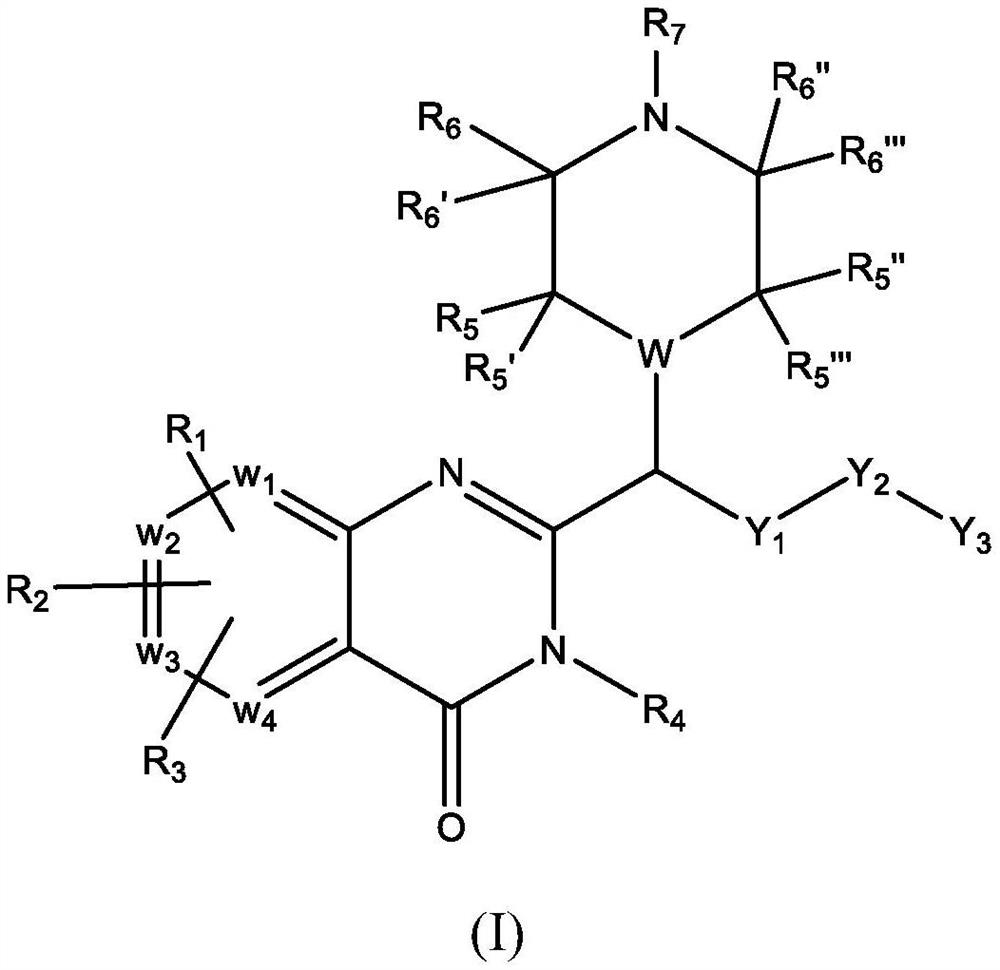
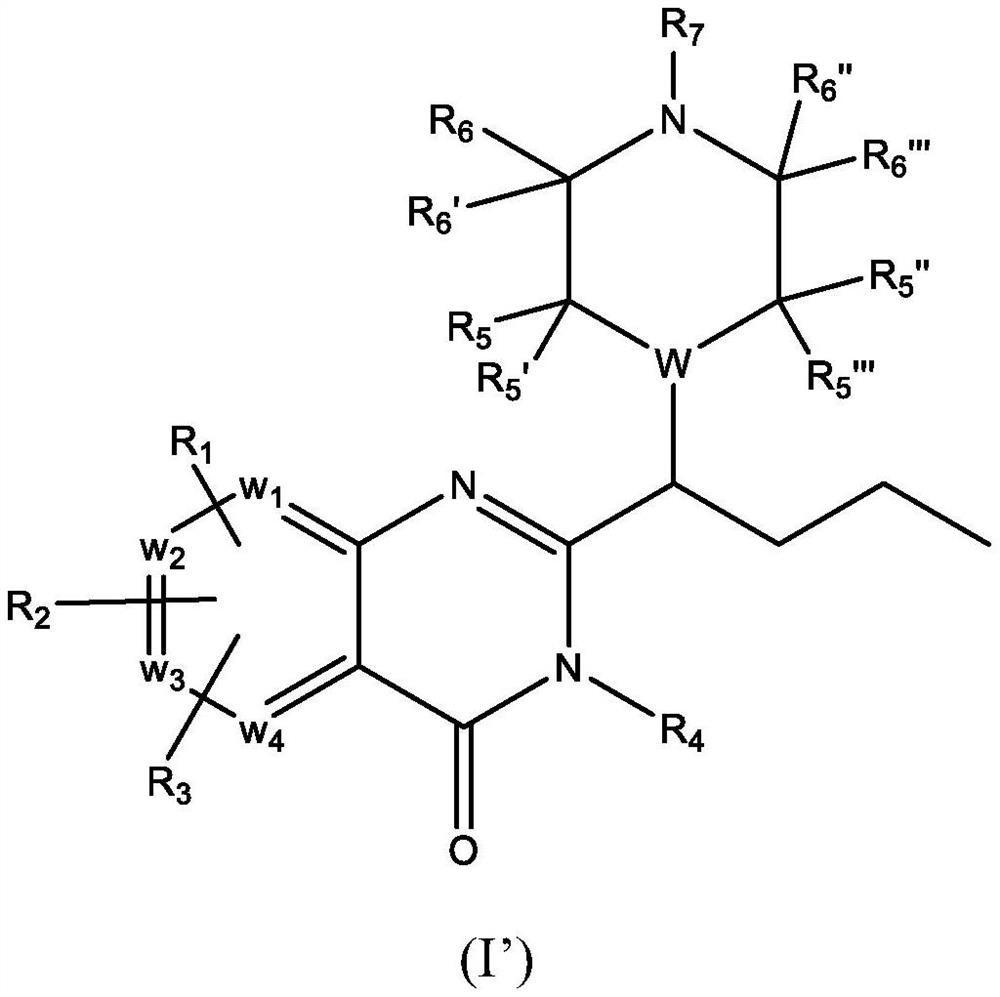
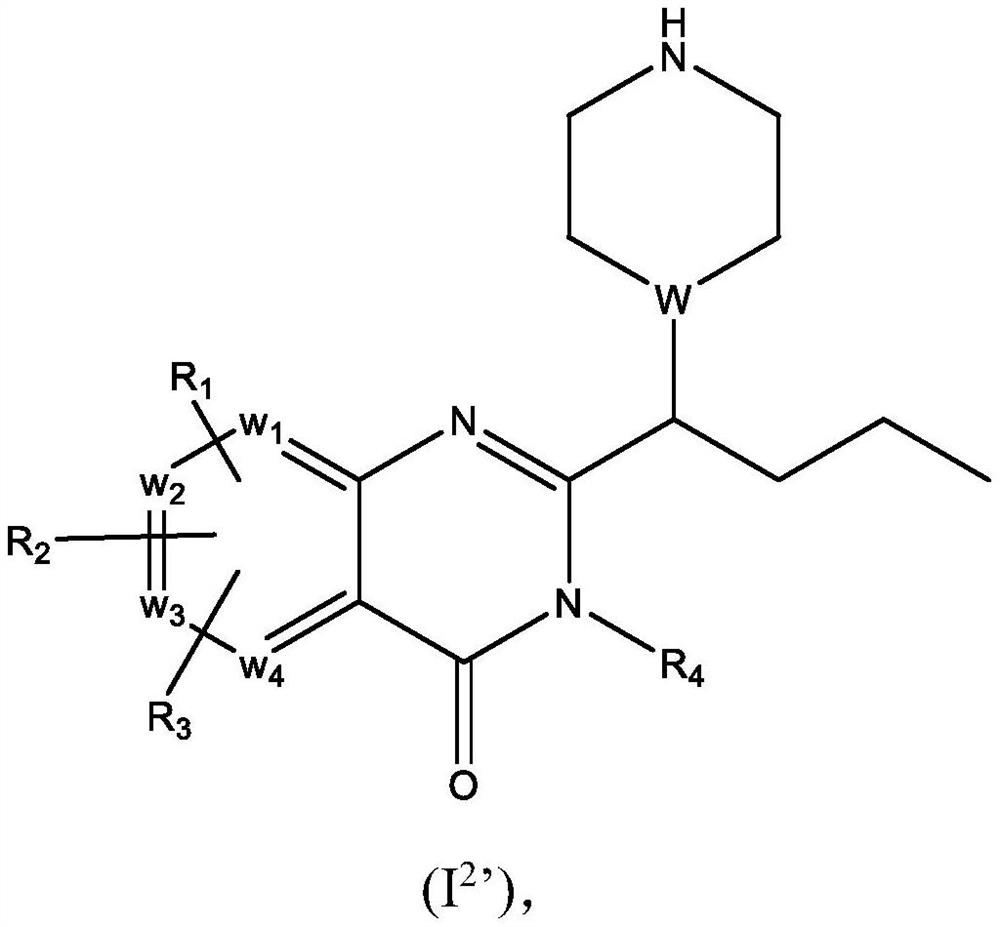
Piperazinyl and piperidinyl quinazolin-4(3H)-one derivatives having activity against pain
An alkyl and alkynyl technology, applied in the field of pain treatment, can solve problems such as drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[1179] The following abbreviations are used in the examples:
[1180] ACN: Acetonitrile
[1181] Aq: water-based
[1182] Anh: anhydrous
[1183] Chx: Cyclohexane
[1184] DCM: dichloromethane
[1185] DIPEA: N,N-Diisopropylethylamine
[1186] DME: Dimethoxyethane
[1187] DMF: Dimethylformamide
[1188] Eq: Equivalent
[1189] Et 2 O: diethyl ether
[1190] EtOAc: ethyl acetate
[1191] h: hours
[1192] HATU: (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate)
[1193] HMDS: Hexamethyldisilazane
[1194] HPLC: High Performance Liquid Chromatography
[1195] LiHMDS: lithium bis(trimethylsilyl)amide
[1196] MeOH: Methanol
[1197] MS: mass spectrometry
[1198] Min: minutes
[1199] Pd(dppf)FeCl 2 : [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II)
[1200] PdCl 2 (bpy): (2,2'-bipyridyl)dichloropalladium(II)
[1201] Quant: quantitative
[1202] Rt.: retention time
[1203] r.t.: room temperature
[...
Synthetic example
[1232]
example 1
[1233] Example 1. 6-bromo-2-(1-((3S,5R)-3,5-dimethylpiperazin-1-yl)butyl)-3-ethylquinazoline-4(3H)- ketone.
[1234] Step a. 2-Amino-5-bromo-N-ethylbenzamide.
[1235] To a solution of 2-amino-5-bromobenzoic acid (10 g, 46.3 mmol) in anhydrous DMF (200 mL) was added TEA (13 mL, 92.6 mmol) and HATU (21.1 g, 55 mmol) under argon atmosphere, And the reaction mixture was stirred at 0 °C for 10 min. Then, ethylamine (2M in THF, 35 mL, 69.4 mmol) was added dropwise, and the reaction mixture was brought to r.t. and stirred overnight. The reaction crude was washed with EtOAc:Et 2 O (300mL, 1:1) diluted with NaHCO 3 Wash with saturated aqueous solution. The organic layer was washed with anhydrous Na 2 SO 4 Dry, filter and concentrate to dryness to give the title compound (10.8 g, yield: 85%).
[1236] Step b. 5-Bromo-N-ethyl-2-pentaneaminobenzamide.
[1237] To a solution of the compound obtained in step a (10.7 g, 44.1 mmol) in anhydrous DCM (200 mL) was added TEA (9.23 mL, 6...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap