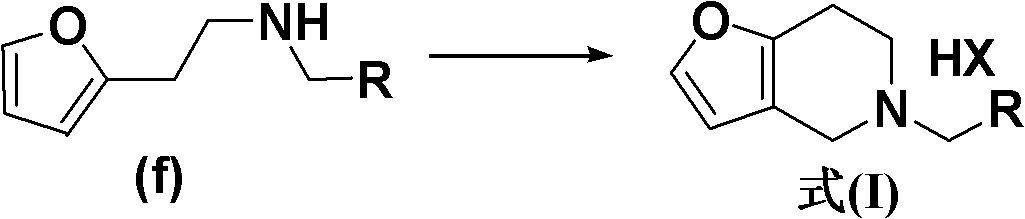
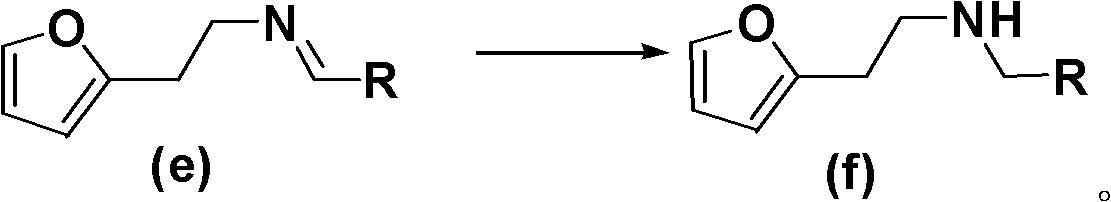
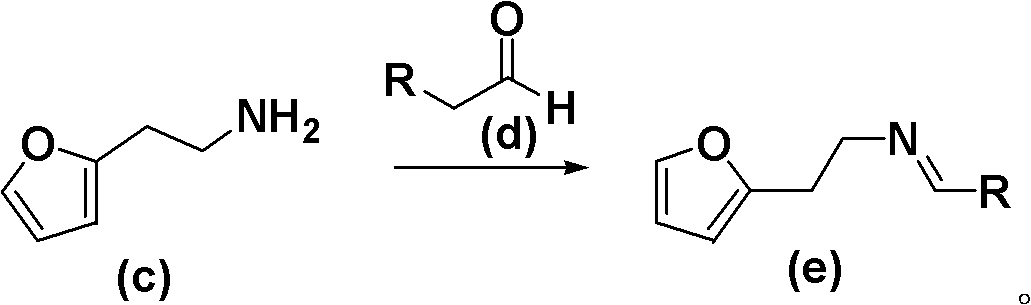
New method for synthesis of substituted furo-piperidine derivatives
A technology of substituents and compounds, applied in the field of preparation of substituted furopiperidine derivatives, can solve the problems of 3-furfural expensive, high production cost, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Step 1: 2-Nitrovinylfuran
[0035]
[0036] Method one: under the condition of -20°C, add furfural (230g), nitromethane (232g) and methanol (800mL) to the reaction flask, mechanically stir, add dropwise methanol (900mL) solution of sodium hydroxide (100g), keep The internal temperature of the reaction system was not higher than -5°C. After the dropwise addition was completed, the reaction was continued at -5°C for 30 minutes. Stop the reaction, add concentrated sulfuric acid (150mL) in methanol (300mL) solution and water (3.6L) to the above system successively, the product is precipitated as a yellow solid, suction filtered and dried to obtain a yellow powdery solid (165g, 49.5%)
[0037] Method two: under the condition of -20°C, add furfural (230g), nitromethane (232g) and methanol (800mL) to the reaction flask, mechanically stir, add dropwise a solution of sodium hydroxide (100g) in water (900mL), keep The internal temperature of the reaction system was not higher...
Embodiment 2
[0061] Example 2 Preparation of 1-cyanoacyl-4-(3-(3,5-difluoro-4-morpholinoanilino)- 6-Methyl)pyrimidinyl-furo[3,2-c]piperidine
[0062] The first step 1-benzyl-4-tributyltin-furo[3,2-c]piperidine
[0063] Under nitrogen protection, 1-benzyl-furo[3,2-c]piperidine (11.2 mg, 1 eq) and dry tetrahydrofuran (150 μL) were added to the reaction flask, and n-butyllithium (16.05 μL, 1eq), continue to add tributyltin chloride (20.8mg, 1.6eq) dropwise, after the addition is complete, continue to react for 2.5 hours. The reaction was stopped, quenched with water, extracted with ethyl acetate (10mL*5), combined the organic phases, dried over anhydrous sodium sulfate, filtered with suction, concentrated, and the crude product was chromatographed on silica gel to obtain 1-benzyl-4-tributyltin-furano [3,2-c]piperidine (19.25mg).
[0064] The second step 1-benzyl-4-(3-chloro-6-methyl)pyrimidinyl-furo[3,2-c]piperidine
[0065] Add 1-benzyl-4-tributyltin-furo[3,2-c]piperidine (3.2 mg, 1 eq),...
Embodiment 3
[0073] Example 3 Compound 1-cyanoacyl-4-(3-(3,5-difluoro-4-morpholinoanilino)-6-methyl)pyrimidinyl-furo[3,2-c]piper In vitro biochemical level inhibition of JAK kinase (PK) activity experiment
[0074] In the biochemical level enzyme activity test, HTRF technology is used to detect the activity of tyrosine kinase. HTRF is a time-resolved fluorescence resonance capacity transfer technology. HTRF (Homogeneous Time-Resolved Fluorescence) is the most commonly used method for detecting analytes in homogeneous systems. This technique combines fluorescence resonance energy transfer (FRET) and time-resolved technology (TR), and has been widely used. Applied to different stages of drug development based on cell experiments and biochemical experiments. According to the measurement principle of HTRF method, after incubating the pure enzyme JAK2 with biotinylated substrate and ATP, add avidin-labeled XL-665 and Eu-labeled antibody that recognizes phosphorylation of the substrate. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com