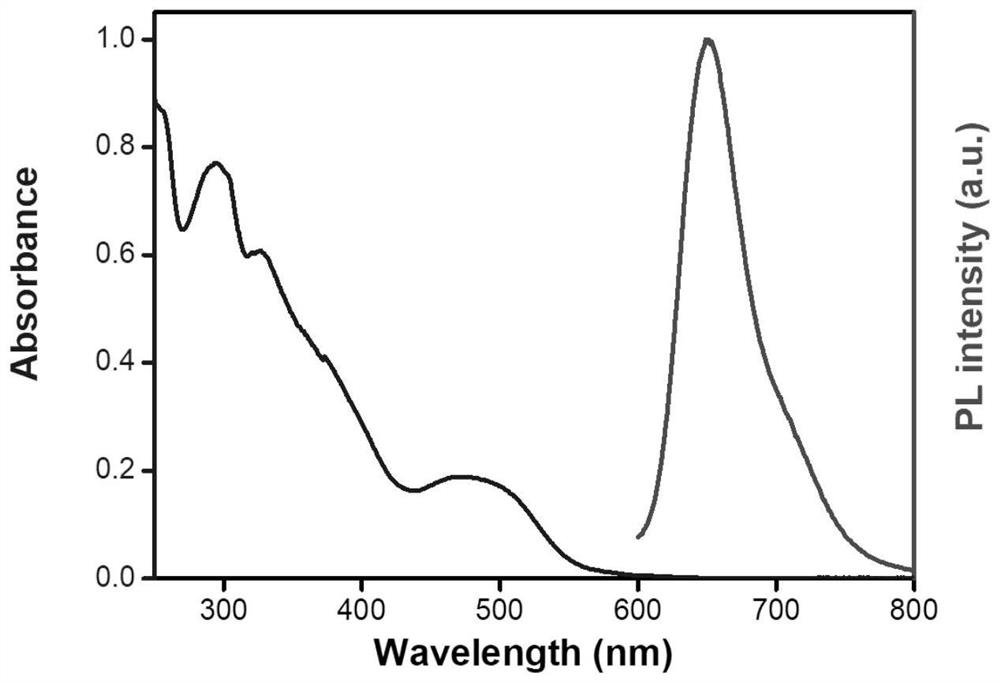
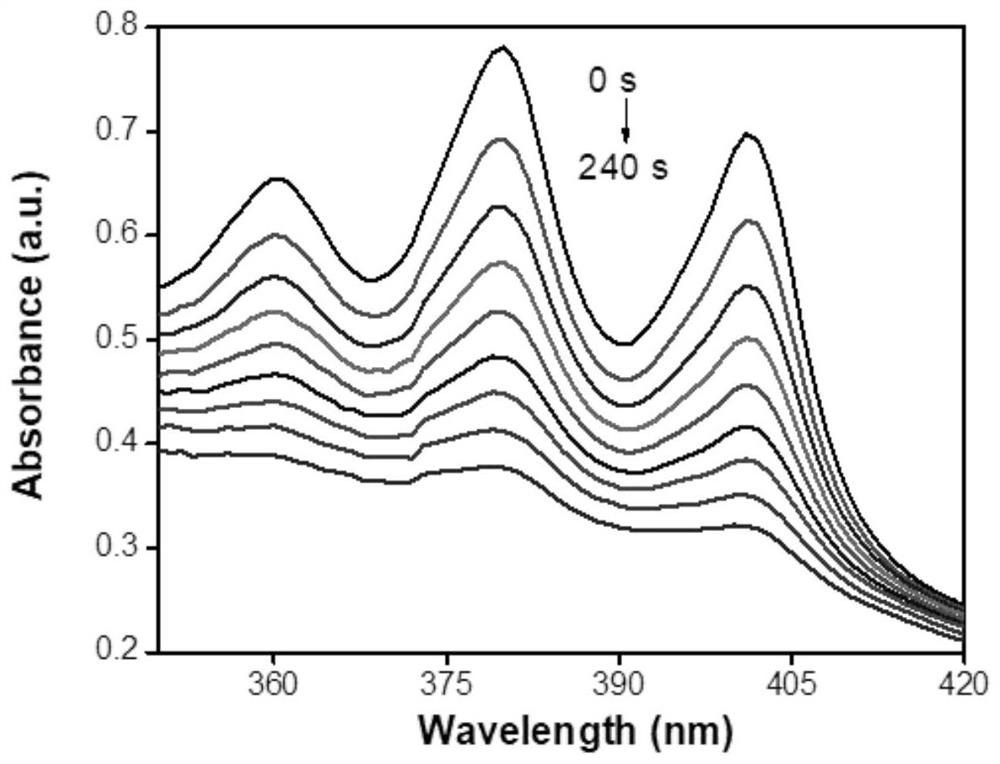
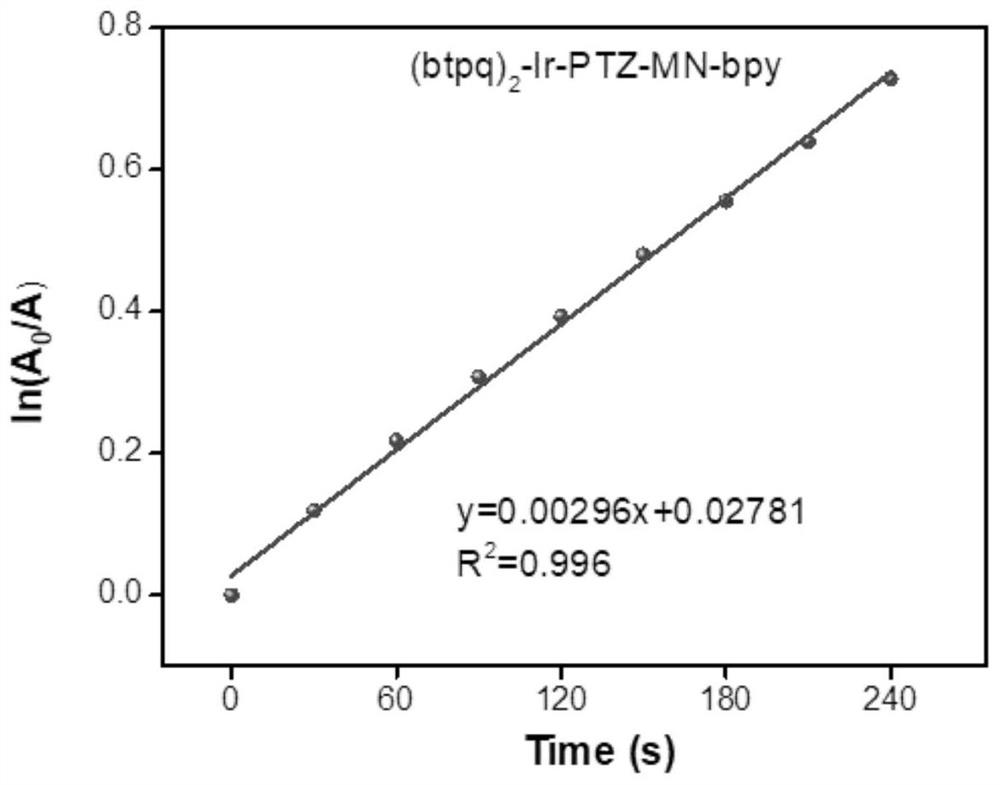
A kind of iridium complex containing phenothiazine and its preparation method and application
A technology of phenothiazine iridium and iridium complexes, which is applied in the field of phenothiazine iridium complexes and their preparation, can solve the problems of low efficiency and achieve strong tissue penetration, low background autofluorescence, and small photodamage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention provides the preparation method of the iridium complex containing phenothiazine described in the above technical scheme, comprising the following steps:
[0043] Mixing phenothiazine, 4-bromobenzonitrile, palladium acetate, tri-tert-butylphosphine, cesium carbonate and the first solvent, and performing a carbon-nitrogen coupling reaction to obtain a compound of the structure shown in formula II;
[0044] Mixing the compound with the structure shown in the formula II, 4-methyl-2,2'-bipyridine-4-carbaldehyde, a base and a second solvent, and performing a condensation reaction to obtain the ligand with the structure shown in the formula III;
[0045] Mix 2-chloroisoquinoline, benzothiophene-2-boronic acid, potassium carbonate, tetrakis(triphenylphosphine)palladium and a third solvent, and carry out a coupling reaction to obtain a ligand of the structure shown in IV;
[0046] Mixing the ligand with the structure shown in the formula IV, iridium trichlo...
Embodiment 1
[0073] Iridium complexes containing phenothiazine [Ir(btpq) 2 (PTZ-MN-bpy)][PF 6 ]Synthesis:
[0074] Weigh 300.0mg of phenothiazine, 292.6mg of 4-bromobenzonitrile, 90.2mg of palladium acetate, 910.8mg of tri-tert-butylphosphine and 651.8mg of cesium carbonate, dissolve in 150mL of toluene, and reflux at 110°C for 24h in a nitrogen atmosphere After the reaction finishes, the gained material is extracted with dichloromethane, and the gained aqueous phase is extracted three times with dichloromethane, and the combined organic phases are dried overnight with anhydrous sodium sulfate, separated by column chromatography (reagent is dichloromethane:petroleum ether) =10:1 (v / v), obtain the ligand of structure shown in formula II (recorded as PTZ-MN);
[0075] Weigh 494.3mg of the ligand (PTZ-MN) and 198.1mg of 4-methyl-2,2'-bipyridine-4-carbaldehyde and 120mg of sodium hydroxide, dissolved in 100mL of ethanol, at room temperature After the reaction, the obtained material was cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com