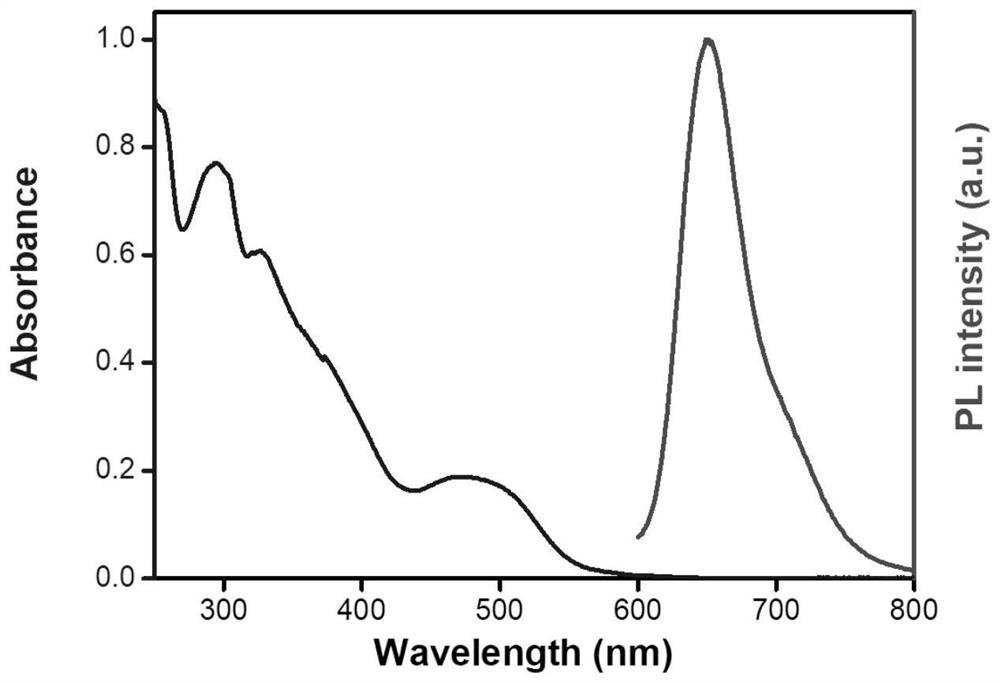
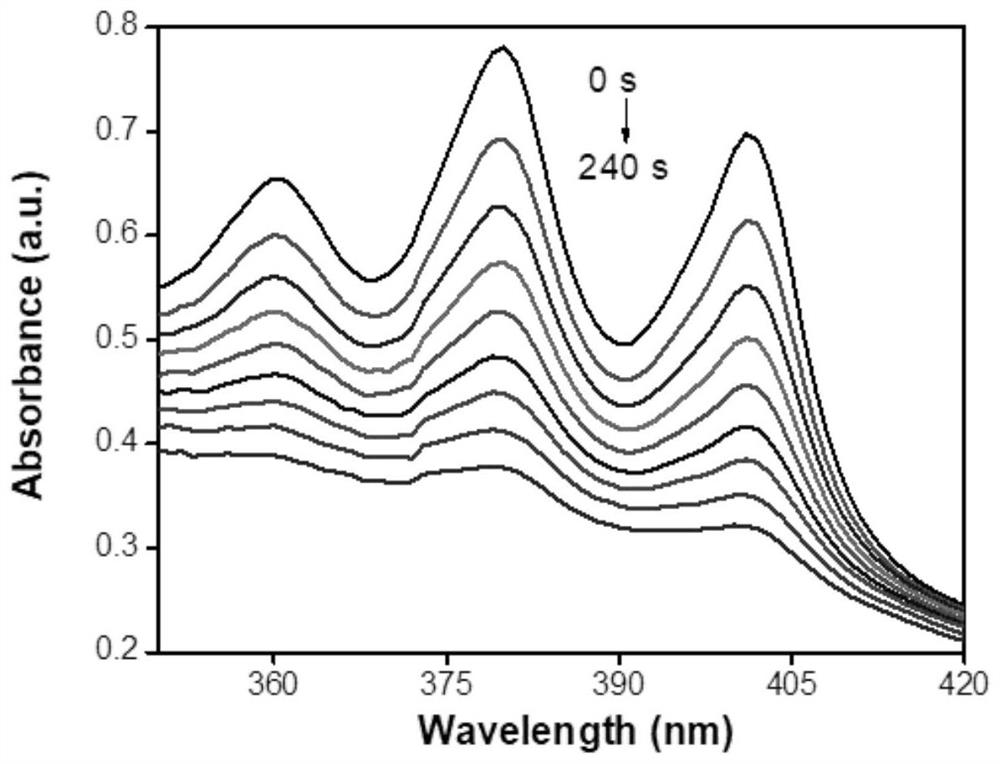
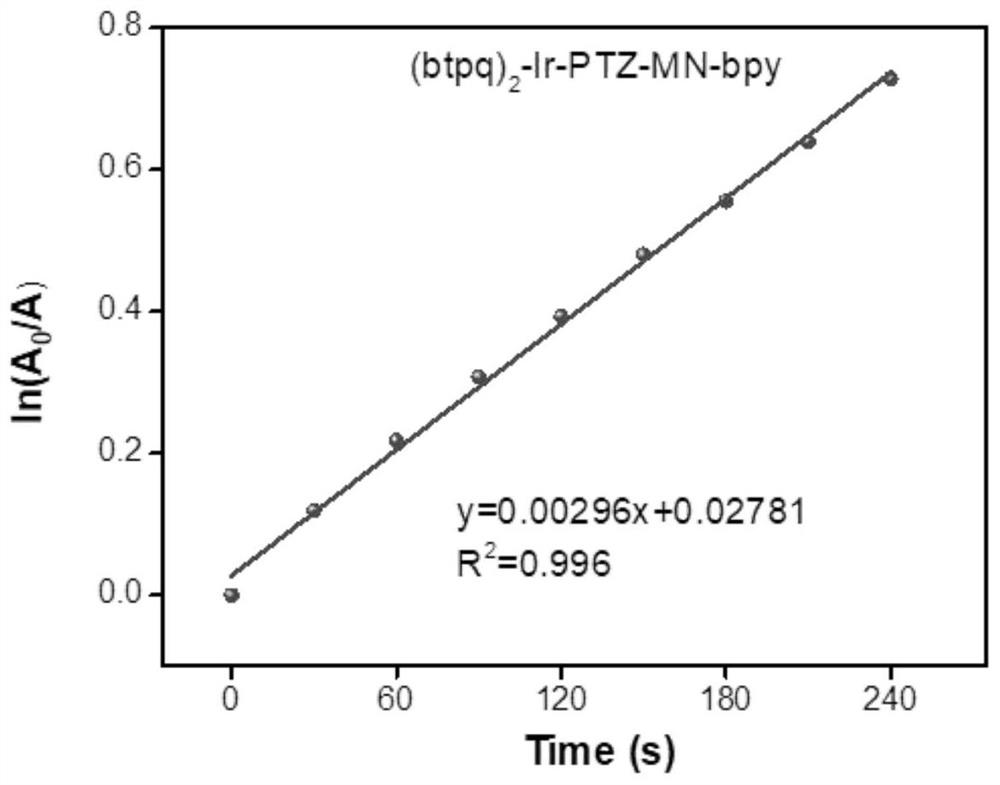
Phenothiazine-containing iridium complex as well as preparation method and application thereof
A technology of phenothiazine iridium and iridium complexes, which is applied in the field of phenothiazine iridium complexes and their preparation, can solve the problems of low efficiency and achieve strong tissue penetration, small light damage, and low background autofluorescence Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention provides the above technical solution containing phenothiazine method for preparing an iridium complex, comprising the steps of:
[0043] Phenothiazine, 4-bromobenzonitrile, palladium acetate, tri-t-butylphosphine, cesium carbonate and the solvent are mixed first, carbonitriding coupling reaction, to give a compound of the structure shown in formula II;
[0044] The compound represented by the structure of formula II, 4- carbaldehyde methyl-bipyridine-2,2'-base and the second solvent are mixed -4-, condensation reaction, to obtain the ligand represented by structural formula III;
[0045] 2-chloro-isoquinoline, benzothiophene-2-boronic acid, potassium tetrakis (triphenylphosphine) palladium and the third solvent are mixed, the coupling reaction, to obtain the ligand structure shown in IV;
[0046] The ligand structure shown by the formula IV, iridium trichloride trihydrate and fourth mixed solvent, a first ligand, to give the structure of iridium compl...
Embodiment 1
[0073] Phenothiazine ruthenium complex [IR (btpq) 2 (PTZ-MN-BPY)] [PF 6 ]Synthesis:
[0074] 300.0 mg of phenothiazine, 292.6 mg of 4-bromine, 90.2 mg of acetate and 910.8 mg of tri-butylphosphine and 651.8 mg of cesium carbonate were dissolved in 150 ml of toluene, 110 ° C in a nitrogen protection atmosphere 24h After the reaction, the resulting material was extracted with dichloromethane to extract the resulting aqueous phase for dichloromethane, combined with an organic phase, dried over the air with anhydrous sodium sulfate, and the column chromatography was separated (the reagent was dichloromethane: petroleum ether) = 10: 1 (v / v), resulting in a ligand of the structure shown in Formula II (PTZ-Mn);
[0075] The ligand (PTZ-Mn) and 198.1 mg of 4-methyl-2,2'-bisin-4-formaldehyde shown in 494.3 mg of formula II were weighed, and sodium hydroxide was dissolved in 100 ml of ethanol, at room temperature. After the reaction was 3 h; After the reaction was completed, the resulting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com